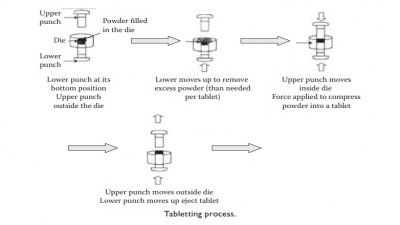
Tablets
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Tablets
The development of a new chemical entity (NCE) requires the testing of its biological activity at various stages of development.
Tablets
Introduction
The
development of a new chemical entity (NCE) requires the testing of its
biological activity at various stages of development. For systemically acting
drugs, animal studies are carried out at early stages of development using
parenteral administration of a solubilized form of the drug. As drug
development proceeds to later stages, human clinical studies are preferred with
an orally administered dosage form that is both simple to formulate and
provides adequate bioavailability. Preferred drug product (DP) dosage form
choices are determined based on the drug substance’s (DS’s) physico-chemical
properties, patient and disease state constraints and preferences, dose,
manufacturability, and commercial factors such as other therapeutic options
available to the patient.
An
oral tablet dosage form is usually the most preferred dosage form because of
patient convenience and acceptance. Most drugs are formulated in tablet dosage
forms. Tablets are available in a wide variety of shapes, sizes, colors, and
surface markings. This chapter would describe the types of tablets and discuss
its formulation components, manufacturing pro-cesses, quality attributes, and
some key considerations in the design and development of an oral tablet dosage
form.
Related Topics