Tetracyclines
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Antibiotics And Synthetic Antimicrobial Agents: Their Properties And Uses
The tetracyclines are a group of broad-spectrum antibiotics that are declining in use as a result of increasing bacterial resistance.
TETRACYCLINES
The tetracyclines are a group of broad-spectrum antibiotics that are
declining in use as a result of increasing bacterial resistance. Despite that,
they remain important antibiotics for several dangerous, but relatively rare,
infections due to chlamydia (e.g. trachoma), rickettsia (e.g. typhus and
Q-fever) and spirochaetes (e.g. Lyme disease) as well as those caused by
‘typical’ bacteria (e.g. brucellosis and bubonic plague). They also represent useful
alternatives to macrolides (see section 4 of this chapter) and to β-lactams
(particularly in cases of allergy) for the treatment of more common infections,
including those of the respiratory tract.
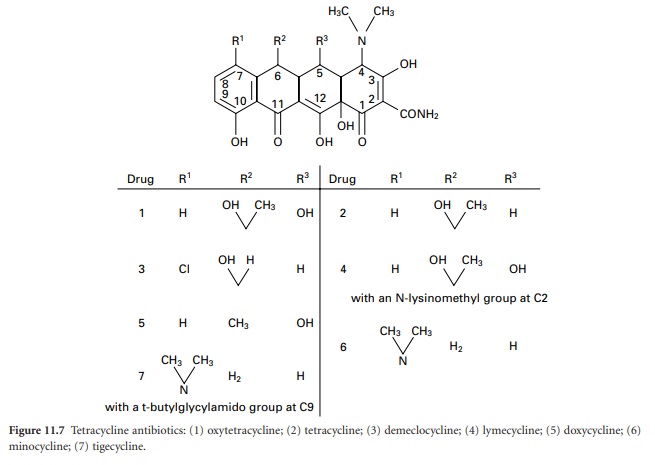
The tetracyclines (Figure 11.7)
were first developed during the 1940s and 1950s and several that are still in
use date from that time, e.g. tetracycline itself, oxytetracycline and
chlortetracycline. Doxycycline and minocycline are more potent semisynthetic
analogues discovered in 1966 and 1972 respectively, after which there were no
significant developments until the introduction, in 2005, of tigecycline (a
glycylglycine derivative), which is generally more potent again than other
tetracyclines and maintains activity against some organisms that have become
resistant to earlier members of the group.
Although the tetracyclines
can exhibit bactericidal activity by inhibiting ribosome function at concentrations that might be used in the laboratory, they are bacteristatic at concentrations that can safely be achieved
in the body. They are active against
Gram-positive bacteria, although many strains of Staph. aureus have become resistant to all but tigecycline which, as a consequence, is of value in the treatment of MRSA infections. Many Gram-negative
species are also sensitive to tetracyclines,
and although the
proportion of strains responding to treatment
has significantly diminished in recent years there are substantial geographical variations in resistance
patterns; Ps. aeruginosa and
Proteus species are normally resistant.
In addition to the infections mentioned above, tetracyclines are prescribed for the treatment of acne, various genital infections, the eradication of Helicobacter pylori in gastric and peptic ulcer
disease (as part
of a multidrug regimen) and,
in the case of doxycycline particularly,
for the prophylaxis of drug-resistant Plasmodium falciparum malaria.
Resistance to the tetracyclines develops relatively slowly, but there is cross-resistance, i.e. an
organism resistant to one member
is usually resistant to all other
members of this group, but there are excep tions: tigecycline (mentioned above),
and tetracyclineresistant Staph. aureus
strains may still
be sensitive to minocycline. Superinfection
(‘overgrowth’) with naturally tetracycline-resistant organisms, for example Candida albicans and other yeasts, affecting the mouth,
upper
respiratory tract or gastrointestinal tract, may occur as a result of the suppression of tetracyclinesusceptible microorganisms.
Tetracyclines are absorbed
from the gastrointestinal tract and oral products are the only form in which they are
currently available in the UK, although ophthalmic, topical and injectable products have been used in the past and
some are still
available in other
countries. Absorption of tetracyclines is inhibited by food,
antacids, milk or products containing dior trivalent cations,
so tetracycline, oxytetracycline and chlortetracycline have to be administered four times a day in order to maintain adequate tissue concentrations. Such impaired absorption is much less
evident in the more recently developed drugs, and partly as a consequence of both this improvement
and of extended half-lives,
lymecycline, minocycline and tigecycline are administered twice daily, and doxycycline just once.
The newer tetracyclines are also more
lipophilic than the early
ones, so doxycycline, and to an even greater extent minocycline, exhibit good
tissue distribution and achieve concentrations in the biliary tract, liver, kidneys and other organs
which may substantially exceed those in the blood.
Dose-dependent nausea
and vomiting are the most common side
effects, and diarrhoea may arise as a consequence of alterations in the bacterial flora of the
colon. The ability
to chelate with calcium results
in tetracyclines being deposited in bones and teeth, and precludes their administration to children younger
than 12 years or to women
in late pregnancy. Their use in patients with
poor kidney function is also contraindicated because most of the tetracyclines accumulate in this situation; again, doxycycline and minocycline are exceptions.
Related Topics
