Agar
| Home | | Pharmacognosy |Chapter: Pharmacognosy and Phytochemistry : Drugs Containing Carbohydrates and Derived Products
It is the dried gelatinous substance obtained by extraction with water from Gelidium amansii or various species of red algae like Gracilaria and Pterocladia, belonging to family Gelidaceae (Gelidium and Pterocladia), Gracilariaceae (Gracilaria).
AGAR
Synonym
Agaragar, Japanese Isinglass, Vegetable gelatin.
Botanical Source
It is the dried gelatinous substance obtained by extraction
with water from Gelidium amansii or
various species of red algae like Gracilaria
and Pterocladia, belonging to family
Gelidaceae (Gelidium and Pterocladia), Gracilariaceae (Gracilaria).
Geographical Source
Japan was the only country producing agar before the World
War II, but it is now produced in several countries like, Japan: Gelidium amnasii and other Gelidium species, Australia; Gracilaria confervoldes, New Zealand; Pterocladia lucida and other allied species, Korea, South Africa, United
States, Chile, Spain, and Portugal.
History
The history of agar and agarose extends back to centuries
and the utility of the compounds closely follow the emer-gence and development
of the discipline of microbiology. The gel like properties of agar are
purported to have been first observed by a Chinese Emperor in the mid sixteenth
century. Soon thereafter, a flourishing agar manufacturing industry was
established in Japan. The Japanese dominance of the trade in agar only ended
with World War II. Following World War II, the manufacturing of agar spread to
other countries around the globe. For example, in the United States, the
copious sea weed beds found along the Southern California coast has made the
San Diego area a hot bed of agar manufacture. Today, the manufacture and sale
of agar is lucrative and has spawned a competitive industry.
Collection
The red algae are grown in rocks in shallow water or on the
bamboos by placing them in the ocean. Collection of the algae is usually made
in summer (May and October). The bamboos are taken out and the seaweeds are
stripped off. Algae are dried, beaten with sticks and shaken to remove the sand
and shell attached to them. Then the entire material is taken to high altitude,
washed with water and bleached by keeping them in trays in the sunlight,
sprinkling water and rotating them periodically. The agar is then boiled; one
part of algae with 50 parts of water acidified with acetic acid or dilute
sulphuric acid. The hot extract is subjected for coarse and fine filtration
using cloth to remove the large and small impurities present in them. The
filtered extract is then transferred into wooden trough which on cooling forms
a jelly like mass. The mass thus obtained is then passed through screw press to
obtain strips of agar. These strips contain water and to remove the water
present in them, the agar strips are placed in open air to get the benefits of
the Japanese climate. During this season, Japan has a very warm day and the
nights are very cold with a temperature less than 0°C. As a result of this
climate the water present on top of the strips are converted into ice at night,
and during day they are reconverted to water and the excess water present in
them are removed. Then, these strips are again dried in the sunlight in trays.
Modern method of deep freezing is being utilized in the
preparation of agar in recent development of technology. The algae which is
collected is washed in running water for a day and then extracted firstly with
dilute acid in steam heated digester and then with water for 30 min, the hot
solution so obtained is cooled and deep freezed in an ice machine. The water
present in the agar is converted to ice and these masses are powdered, melted
and filtered in rotary vacuum filter. The moist agar is dried using dry air and
powdered agar is obtained.
Morphology

Chemical Constituents
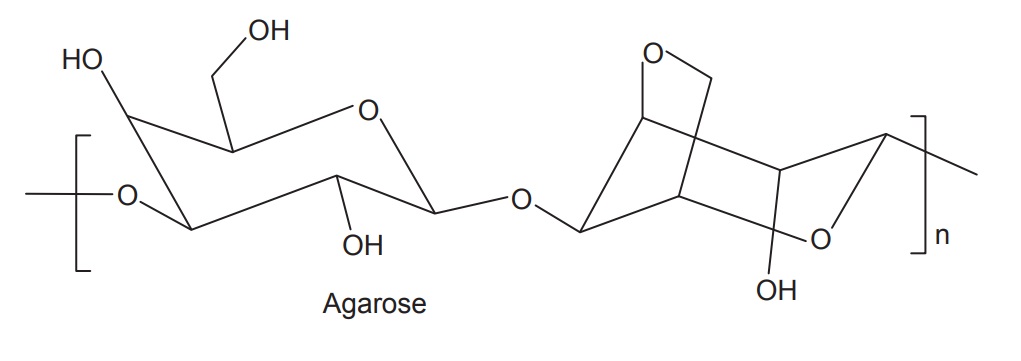
Agar is a complex heterosaccharide and contains two
dif-ferent polysaccharides known as agarose and agaropectin. Agarose is neutral
galactose polymer and is responsible for the gel property of agar. It consists
of D-galactose and L-galactose unit. The structure of agaropectin is not
completely known, but it is believed that it consists of sulphonated
polysaccharide in which galactose and uronic acid are partly esterified with
sulphuric acid. Agaropectin is responsible for the viscosity of agar solution.
Chemical Tests
1. Agar responds positively to Fehling’s solution test.
2. Agar gives positive test with Molisch reagent.
3. Aqueous solution of agar (1%) is hydrolysed with con-centrated HCl by heating for 5–10 min. On addition of barium chloride solution to the reaction mixture, a white precipitate of barium sulphate is formed due to the presence of sulphate ions. This test is absent in case of starch, acacia gum and tragacanth.
4. To agar powder a solution of ruthenium red is added. Red colour is formed indicating mucilage.
5. Agar is warmed in a solution of KOH. A canary yellow colour is formed.
6. An aqueous solution of agar (1%) is prepared in boiling water. On cooling it sets into a jelly.
7. To agar solution an N/20 solution of iodine is added. A deep crimson to brown colour is obtained (distinctive from acacia gum and tragacanth).
8. To a 0.2% solution of agar an aqueous solution of tannic acid is added. No precipitation is formed indicating absence of gelatin.
9. Agar is required to comply with tests for the absence of E. coli and Salmonella, and general microbial contamination should not exceed a level of 103 microorganisms per gram as determined by a plate count. It has a swelling index of not less than 10.
Uses
Agar is used to treat chronic constipation, as a laxative, sus-pending agent, an emulsifier, a gelating agent for suppositories, as surgical lubricant, as a tablet excipient, disintegrant, in production of medicinal encapsulation and ointment and as dental impression mold base. It is extensively used as a gel in nutrient media for bacterial cultures, as a substitute for gelatin and isinglass, in making emulsions including photographic, gel in cosmetic, as thickening agent in food especially confectionaries and dairy products, in meet canning; sizing for silk and paper; in dying and printing of fabrics and textiles; and in adhesive.
Substitutes and Adulterants
Some of the common adulterants present in agar are gelatin and Danish agar. The presence of gelatin can be detected by addition of equal volume of 1% trinitrophenol and 1% of agar solution; the solution produces turbidity or precipitation. Danish agar has an ash of 16.5–18.5%, it is formed from rhodophyceae indigenous to the Denmark costal region. The Danish agar has a gel strength which is half of its gel strength of Japanese agar.
