Classification - Drugs Containing Tannins
| Home | | Pharmacognosy |Chapter: Pharmacognosy and Phytochemistry : Drugs Containing Tannins
The tannin compounds can be divided into two major groups on the basis of Goldbeater’s skin test. A group of tannins showing the positive tanning test may be regarded as true tannins.................
CLASSIFICATION
The tannin compounds can be divided into two major groups on
the basis of Goldbeater’s skin test. A group of tannins showing the positive
tanning test may be regarded as true tannins, whereas those, which are partly
retained by the hide powder and fail to give the test, are called as
pseudotannins.
Most of the true tannins are high molecular weight
compounds. These compounds are complex polyphenolics, which are produced by polymerization
of simple polyphenols. They may form complex glycosides or remains as such
which may be observed by their typical hydrolytic reaction with the mineral
acids and enzymes. Two major chemical classes of tannins are usually recognized
based on this hydrolytic reaction and nature of phenolic nuclei involved in the
tannins structure. The first class is referred to as hydrolysable tannins,
whereas the other class is termed as condensed tannins.
Hydrolysable Tannins
As the name implies, these tannins are hydrolysable by
mineral acids or enzymes such as tannase. Their structures involve several
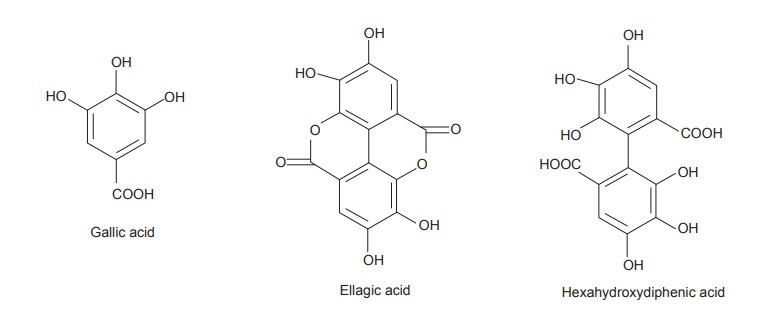
molecules of polyphenolic acids such as gallic, hexahydrodiphenic, or ellagic
acids, bounded through ester linkages to a central glucose molecule. On the basis
of the phenolic acids produced after the hydrolysis, they are further
categorized under gallotannins composed of gallic acid or ellagitannins which
contains hexahydrodiphenic acid which after intraesterification produces
ellagic acid.
Hydrolysable tannins are sometimes referred to as pyrogallol
tannins as the components of phenolic acids on dry distillation are converted
to pyrogallol derivatives. The hydrolysable tannins are soluble in water, and
their solution produces blue colour with ferric chloride.
Nonhydrolysable or Condensed Tannins
Condensed tannins, unlike the previously explained group are
not readily hydrolysable to simpler molecules with mineral acids and enzymes,
thus they are also referred to as nonhydrolysable tannins. The term
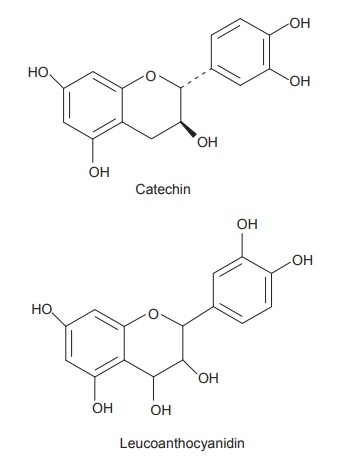
proanthocyanidins is sometimes alternatively used for these tannins. The
compounds containing condensed tannins contain only phenolic nuclei which are
biosynthetically related to flavonoids. Catechin which is found in tannins is
flavan-3-o1, whereas leucoanthocyanidins are flavan-3,4-diol structures. These
phenolics are frequently linked to carbohydrates or protein molecules to
produce more complex tannin com-pounds. When treated with acids or enzymes,
they tend to polymerize yielding insoluble red coloured products known as
phlobaphens. The phlobaphens give characteristic red colour to many drugs such
as cinchona and wild cherry bark. On dry distillation, they yield catechol
derivatives. Condensed tannins are also soluble in water and produces green
colour with ferric chloride.
The families of the plants rich in both of the above groups
of tannins include Rosaceae, Geraniaceae, Leguminosae, Combretaceae,
Rubiaceae, Polygonaceae, Theaceae, etc. The members of families Cruciferae and
Papaveraceae on the other hand are totally devoid of tannins. In the plants in
which tannins are present, they exert an inhibitory effect on many enzymes due
to their nature of protein precipitation and therefore contribute a protective
function in barks and heartwood.
Pseudotannins
Pseudotannins are simple phenolic compounds of lower
molecular weight. They do not respond to the tanning reaction of Goldbeater’s
skin test. Gallic acid, Chlorogenic acid, or the simple phenolics such as
catechin are pseudotannins which are abundantly found in plants, especially in
dead tissues and dying cells.
