Efficacy From Published Trials
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: The Efficacy and Safety of Selective Serotonin Reuptake Inhibitors for the Treatment of Depression in Children and Adolescents
The studies used many outcome measures including (1) symptom rating scale score change from baseline (intent to treat model); (2) symptom rating scale score change at final endpoint (completers model); (3) percent improved and (4) clinical global impression (CGI) rating score change.
EFFICACY FROM PUBLISHED TRIALS
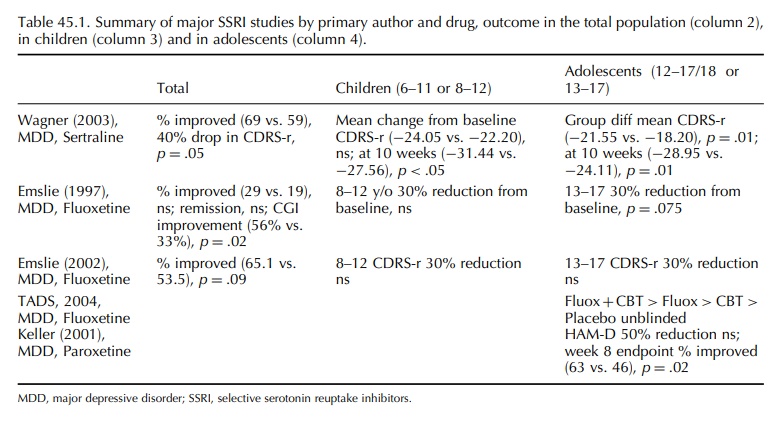
Table
45.1 describes the major published SSRI efficacy studies conducted in US youth.
The studies used many outcome measures including (1) symptom rating scale score
change from baseline (intent to treat model); (2) symptom rating scale score
change at final endpoint (completers model); (3) percent improved and (4)
clinical global impression (CGI) rating score change. Two of the fluoxetine
studies did not meet the a priori primary endpoint of symp-tom score reduction
from baseline (Emslie, Rush and Weinberg, 1997; Emslie et al., 2002) causing a FDA statistical expert to reject the
efficacy claim (Shen, 2003). For the studies including both children and
adolescents, Wagner (sertraline) and Emslie (fluoxe-tine), significant
improvement resided entirely in the adolescent group. Moreover, even in the age
group most likely to benefit, symptom reduction was not different between active
drug- and placebo-treated youth in the paroxetine study of adolescent
depression by Keller et al. (2001).
The authors therefore based the conclusion of paroxetine efficacy on a
relatively modest difference in the percent improved (63 vs. 46). The NIMH-funded
treatment of adolescent depression study (TADS) was added to the list of
registra-tion trials for the FDA safety analysis (see below ‘SAFETY FROM
CLINICAL TRIAL DATA’). The TADS study is listed here among the major efficacy
studies, but it should be noted that it was primar-ily conducted to assess the
efficacy of fluoxetine relative to a psychotherapy intervention of proven
efficacy [cognitive behaviour therapy (CBT)] and may better qualify as a
management trial because of the limited use of blinded observations. The TADS
authors concluded that for the treatment of adoles-cent depression fluoxetine
in combination with CBT gave better results than fluoxetine alone, CBT alone or
placebo. In summary, published US studies on SSRIs show modest but statistically
significant effects over placebo in adolescents. The data do not support
effi-cacy in children although published clinical interpreta-tions suggest
moderate overall effectiveness (Vitiello and Swedo, 2004), are silent on this
important distinc-tion (Cheung, Emslie and Mayes, 2005) and focus on the weak
safety evidence in the face of the seri-ous risks of the failure to treat
depression irrespective of efficacy in children compared with adolescents
(Brent, 2004; Cheung, Emslie and Mayes, 2005; Mann et al., 2006).
Related Topics
