Electrophysiological Basis of Torsade De Pointes
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Withdrawal of Terodiline: A Tale of Two Toxicities
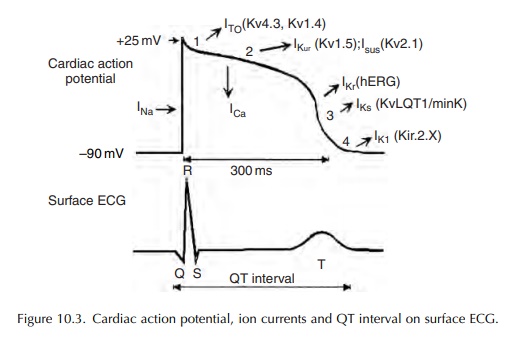
Prolonged ventricular repolarization and subsequent QT interval prolongation result most frequently from a reduction in outward repolarizing potassium current.
ELECTROPHYSIOLOGICAL BASIS OF
TORSADE DE POINTES
Prolonged
ventricular repolarization and subsequent QT interval prolongation result most
frequently from a reduction in outward repolarizing potassium current. However,
in rare instances, these could also result from enhanced or sustained
depolarizing inward sodium or calcium currents (Figure 10.3).
Two hypotheses have been proposed to explain the electrophysiologic mechanisms underlying the induction of torsade de pointes (Surawicz, 1989).
One
hypothesis postulates a trigger mechanism, while the other has re-entry as its
basis. However, it now appears that the two hypotheses are not mutu-ally
exclusive, but may in fact be complementary (Figure 10.4) (Antzelevitch, 2004).
Against
a background of prolonged QT interval, the presence of a slow heart rate gives
rise to early after-depolarizations (EADs), mediated by slow inward calcium
current during the late phase 2 of the action potential. The amplitude of these
EADs is cycle length dependent, with a strong correlation between the preceding
RR interval and the amplitude of EAD that follows. When these EADs reach a
critical thresh-old, they trigger an ectopic beat that initiates torsade de
pointes (Figure 10.4).
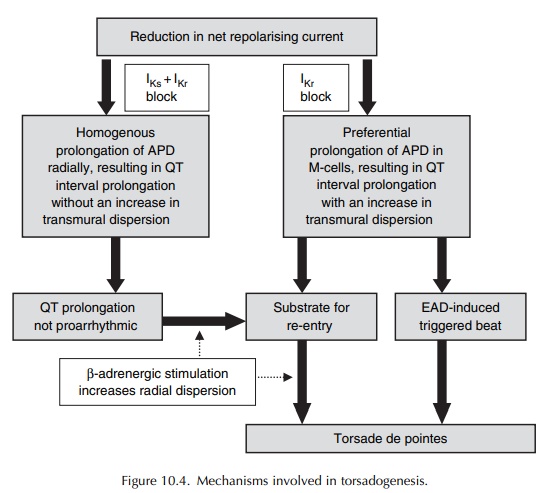
A ventricular cell subtype designated the M-cell, which is found in the deep sub-epicardial to mid-myocardial layers, is very sensitive to the effects of IKr blockers. These cells, also found in human ventricles, have electrophysiological properties that are different from those of epicardial or endocardial ventricular cells, and intermediate between those of the ventricular muscle and the Purkinje fibres. Rela-tive to the epicardial and endocardial myocytes, these M-cells are characterized by (i) the weak presence of the slowly activating component of the repolariz-ing potassium current IKs and (ii) the presence of the more sustained depolarizing slow sodium INa and calcium ICa currents. Another hallmark of these M-cells is the ability of their action potential to lengthen markedly with decreasing stimulation rate.
Since
the repolarizing IKs is weak, these M-cells rely almost exclusively
on the presence of fully functional IKr for repolarization. All
these differences render the M-cells more susceptible to the effects of IKr
block, which thereby respond with a more prolonged action potential and
induction of EADs. Not surpris-ingly, IKr blockers have profound
effect in these cells, giving rise not only to prolongation of the QT inter-val
on the surface ECG, but also to an increase in transmural dispersion of
repolarization (radially) across the myocardial wall at tissue level. An
increase in transmural dispersion of repolarization creates an
electrophysiological environment, or gradient, for the development of re-entry
(Figure 10.4). This radial dispersion of repolarization, rather than QT
interval prolongation, is now widely regarded as both the proarrhythmic
substrate and a more predictive and reliable marker of the proarrhythmic risk
(Fenichel et al., 2004; Antzelevitch,
2005).
As
a corollary, drugs that block both IKr and IKs (and other
relevant ion channels and receptors) may be expected to uniformly prolong the
action poten-tial across the entire thickness of the ventricular wall (and
therefore, the QT interval), without having any significant effect on
transmural dispersion of repolar-ization. Although these agents (e.g.
amiodarone and the recently developed antianginal drug ranolazine) prolong the
QT interval, they have not been found to be proarrhythmic.
Related Topics
