HIV Treatment Guidelines
| Home | | Pharmacology |Chapter: Essential pharmacology : Antiviral Drugs
The treatment of HIV infection and its complications is complex, prolonged, needs expertise, strong motivation and commitment of the patient, resources and is expensive.
HIV TREATMENT GUIDELINES
The treatment of HIV infection and its complications is complex,
prolonged, needs expertise, strong motivation and commitment of the patient,
resources and is expensive. Antiretroviral therapy is only 20 years old, and
strategies are still evolving. Initially, anti-HIV drugs were used singly one
after the other as each failed in a patient due to emergence of resistance.
Understanding the biology of HIV infection and availability of several potent
drugs belonging to different classes has mandated ‘highly active anti-retroviral
therapy’ (HAART) with combination of 3 or more drugs whenever indicated.
Mono-therapy is contraindicated.
It has been realized that even with HAART, which rapidly kills >
99% virions, a small number survive within the resting CD4 lymphocytes and
invariably give rise to relapse when treatment is discontinued despite complete
absence of detectable viraemia and normal CD4 cell count for years. Moreover,
HIV reverse transcriptase is highly copying error prone, implying that viral
replication produces changes at some base pairs (and codons) with high frequency—rate
of mutation is high. Some mutations confer resistance to one or the other
antiretroviral drugs. The resistant mutants are selected by anti-HIV therapy
and in time an apparently sensitive population is replaced by resistant virions.
As the disease progresses in the individual (and several anti-HIV drugs are
used) the HIV population becomes genetically complex and diverse with respect
to susceptibility to drugs. Each failing regimen limits future treatment
options. Even primary drug resistance (i.e. in untreated patients) is being
detected in ~10% HIV patients.
Since none of the
currently available regimens can eradicate HIV from the body of the patient,
the goal of therapy is to maximally and durably inhibit viral replication so
that the patient can attain and maintain effective immune response towards
potential microbial pathogens. Greater the suppression of viral replication,
lesser is the chance of emergence of drug resistant virus.
Initiating Antiretroviral Therapy
Although it is attractive to treat all symptomatic and
asymptomatic HIV positive patients, no long-term clinical benefit has been
demonstrated in asymptomatic cases with reasonable immune competence (CD4 cell
count > 350/μl). Arguments against early treatment in asymptomatic stable
patients include—deleterious effect of antiHIV drugs on quality of life, their
side effects and toxicity, especially lipid abnormalities, drug interactions,
risk of drug resistance limiting future treatment options, limited durability
of available regimens, risk of dissemination of resistant virus and high cost.
The best time to initiate antiHIV therapy remains uncertain. Various
professional bodies and health authorities have framed treatment guidelines
from timetotime. A summary of current concensus is presented below.
1. CD4 cell count is
the major determinant of initiating therapy in asymptomatic cases. Increased
mortality occurs when treatment is begun after CD4 count has fallen below 200/μl, because the patient
is at high risk of serious opportunistic infection, and response to antiHIV
drugs is suboptimal.
2. All cases of
symptomatic HIV disease—treatment recommended.
3. Asymptomatic HIV
disease with CD4 count < 200/μl —treatment recommended.
4. Asymptomatic HIV
disease with CD4 count > 200/μl —treatment decision to be individualized
based on:
·
CD4 cell count and rate of decline: Most
authorities agree that patients with > 350 CD4 cells/μl need not be treated.
Thus, treatment may be initiated at CD4 count between 200–350/μl depending on other
considerations. A decline of > 100 CD4 cells/μl per annum is
considered high—and an indication for initiating therapy.
·
HIVRNA level: > 50,000 copies of HIVRNA/ml
is considered high. However, many authorities recommend treatment in patients
even with > 20,000 copies/ ml.
·
Patient’s interest and potential to adhere to
therapy.
·
Individual risks of drug toxicity and
interactions.
Therapeutic Regimens
Whenever treatment is instituted, it should be aggressive (HAART)
aiming at suppressing plasma viral load to undetectable levels (< 50 copies
of HIVRNA/ml). Therapy with 3 antiretroviral drugs is considered optimal.
Addition of a 4th drug to treatment-naïve patients affords no additional
benefit; may be tried in failed patients. Due to availability of multiple
drugs, a variety of combination regimens are possible and have been employed.
However, no specific combination can be considered optimal initial regimen for
all patients. Choice has to be made on the basis of efficacy, durability,
tolerability, convenience, drug interactions, impact on future options and
cost.
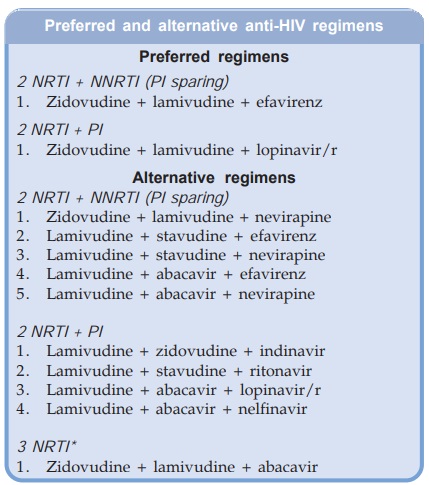
Some of the preferred
and alternative regimens for initial treatment (employing ARV drugs currently
available in India) are given in the box. The important general points are:
· The 3 drugs in the regimen should belong to at
least 2 different classes. Single class regimens are inferior. There is
convincing evidence that 3NRTI regimens are clinically less effective than
those which include a NNRTI or a PI.
· The 3 NRTI regimen is employed only when a
NNRTI or PI cannot be used; such as in patients receiving rifampin or other
interacting drugs.
· The PI sparing regimens (2 NRTI + NNRTI) are
more convenient with lower pill burden, simpler dosing schedules, more
acceptable, better tolerated and produce less metabolic complications; are
preferred by many.
·
The 3 class regimen (NRTI + NNRTI + PI) is
reserved for advanced cases who have failed multiple earlier regimens, so as to
avoid multiclass resistance and higher toxicity.
·
If drug toxicity develops, either the entire
regimen should be interrupted or the offending drug should be changed. No dose
reduction should be tried.
·
‘Drug holidays’ or ‘structured treatment
interruptions’ may briefly improve well being (by absence of side effects), but
allow viral replication and increase risk of drug resistance; are not recommended.
·
Treatment is practically lifelong.
·
Pregnancy in women does not contraindicate
antiHIV therapy. Drugs considered relatively safe during pregnancy are:
Zidovudine, lamivudine, nevirapine, nelfinavir, saquinavir.
·
The ARV drug combinations that should not be
employed are given in the box.
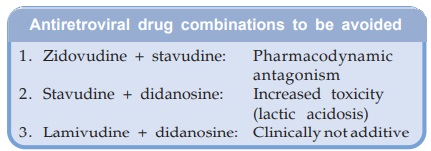
Antiretroviral drug combinations to be avoided
When indicated, PEP should be started as soon as possible,
preferably within 1–2 hours of exposure. The likelihood of preventing infection
declines with the delay; some guidelines do not recommend starting it beyond 72
hours of exposure. According to others, in case of default, PEP may even be
started even 1–2 weeks later. Though HIV infection may not be prevented, onset
of AIDS may be delayed by the late-start PEP.
Prophylaxis Of HIV Infection
Perinatal prophylaxis HIV may be transmitted from the mother to the child either through
the placenta, or during delivery, or by breastfeeding. The highest risk of
transmission is during the birth process. In HIV positive women who are not
receiving ART, zidovudine (300 mg BD) started during 2nd trimester and
continued through delivery to postnatal period, with treatment of the neonate
for 6 weeks has been found in clinical trials to reduce the chances of mother to child
transmission by 2/3rd. Combination therapy is even more effective. Even if not
started earlier, zidovudine administered during labour and then to the infant
is also substantially protective. Many obstetricians offer 3 drug ART to HIV
positive asymptomatic women after the 1st trimester. Breastfeeding by HIV
positive mother should be discouraged, as it may transmit the virus to the
infant.
