Pharmionics in Overview
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Introduction to Pharmionics
This topic, if one takes a broad view, is one of many aspects of pharmacotherapeutics that was largely neglected until relatively recently.
PHARMIONICS IN OVERVIEW
This
topic, if one takes a broad view, is one of many aspects of
pharmacotherapeutics that was largely neglected until relatively recently. A
major reason for neglect of patient adherence was the poor state of available
methods for compiling drug dosing histories in ambulatory patients. Sometimes
called ‘external drug exposure’, reliable drug dosing histories are the
cornerstone of understanding how prescribed drugs are actually being used by
ambulatory patients. That understanding, in turn, is the foundation for
under-standing the clinical and economic consequences of observed patterns of
drug usage/misusage. Thus, the qualities of methods for compiling drug dosing
histo-ries of ambulatory patients are a natural topic of this chapter. So too
are the methods of analysing the clini-cal and economic consequences of
variable adherence to prescribed drug dosing regimens.
DESCRIPTIVE ASPECTS
Three
basic patterns characterize the main devia-tions from prescribed drug dosing
regimens. Some patients – usually in the range of 5%–10%, but sometimes more or
sometimes less – never start the prescribed course of drug dosing. This pattern
is called ‘nonacceptance’. It is shown by the abrupt drop at time zero in the
percentage of patients engaged with the drug dosing regimen, the line labelled
‘persis-tence’ in Figure 48.1. These are patients who never start the dosing
regimen, though have enrolled in the treatment programme. They may take an
initial dose or two, but most of them take none, and then disap-pear from the
treatment programme. There may be a time that they come back to treatment, but
it does not fall within the duration of the study or treatment plan in
question.
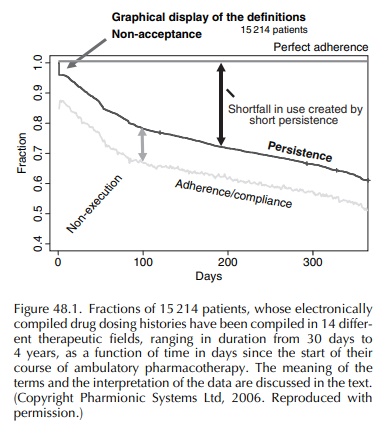
Once the patient engages with the drug dosing regi-men, there is an ongoing question of the quality of the patient’s execution of that regimen. The main errors that patients make in execution are to delay or omit doses. Sometimes they sequentially omit multi-ple scheduled doses, which are called ‘drug holidays’ when they exceed 3 days’ duration. Occasionally, some patients take an extra dose, but missed doses generally outnumber extra doses by 4:1 or more. On any given day, within a group of patients still engaged with the dosing regimen, about 10% of prescribed doses are not taken, giving rise to the gap, seen in Figure 48.1, between the ‘persistence’ line and the lower, somewhat irregular line, labelled ‘adher-ence/compliance’ – the irregularities being due to day-to-day variations in the proportion of prescribed doses that are missed. Within that gap, of course, lie some important details, the first of which is that most of the gap arises from dose omissions made by about a third of ambulatory patients (Urquhart, 1997), and of course includes drug holidays, most of which are taken by a small minority of patients, although within 6 months about half of patients monitored in the stud-ies that comprise Figure 48.1 had had at least one holiday. The third major deviation from prescribed drug dosing regimens is early cessation of dosing, such that dosing stops, and remains stopped without resumption within the time frame of the study or clinical situation.
Figure
48.1 illustrates the foregoing points. Follow-ing the immediate drop due to
non-acceptors, we are left with patients who engage with the dosing regi-men.
They dwindle in numbers throughout the one-year period shown in Figure 48.1. By
the end of the first year, in the 15 214-patient cohort represented by Figure
48.1, about a third had discontinued what was meant to be multi-year, if not
lifetime, treat-ment. Note the large gap between the ‘persistence’ line and the
‘perfect adherence’ line. This gap, which grows with time, indicates both the
loss of patients from beneficial treatment, with its implications for public
health, and the loss of sales revenues for the drug
developer/manufacturer/marketer. When one sees year-by-year growth in revenues
from a pharma-ceutical indicated for long-term use, it signifies that the
product’s marketing effort must not only recruitreplacements for the
non-persisters, but also recruit additional patients. That process of intensive
recruit-ment of new patients continues year after year. Some analysts refer to
this costly and inherently wasteful process as ‘churn’, the high costs of which
could be reduced if the gap between actual and perfect persis-tence could be
narrowed.
One
can expect to see variation within the above numbers, from one treatment
situation to another, but the basic patterns of non-acceptance, incomplete execution
and early discontinuation are pervasive in long-term ambulatory
pharmacotherapy. To illustrate one end of the range of variation, Catalan and
LeLo-rier studied the persistence of Canadian patients with prescribed drugs of
the statin category, following the patients for 5 years after they were
prescribed a statin. Each patient’s drugs were fully reimbursed, which means
that economic obstacles to continuity of treat-ment were nullified. Switches
between one drug and another within the ‘statin’ class were considered to
represent continuity of statin treatment. Following are the percentages of
patients still persisting from the first to the fifth anniversary of the
original prescrip-tion: 33, 24, 17, 14, 13. This pattern shows twice the loss
of patients within the first year as shown in Figure 48.1. Perhaps the reasons
for this exceptionally high rate of discontinuation in the Catalan–LeLorier
study lie in the fact that the patients in this study were on full social
assistance, which means that they were eligible for economic support by the
state, in addition to getting prescription drugs at no cost. The various
problems that led these patients to qualify for full social assistance may
include factors that espe-cially discourage long persistence with chronic-use
medicines for asymptomatic conditions.
To
illustrate the other end of the range of variation, the big confirmatory trials
of several major drugs of the statin class show that over 90% of patients
enrolled in the studies were continuing to attend scheduled clinic visits, and
presumably were still taking the trial medication at some level of
adherence/compliance (Scandinavian Simvastatin Survival Study Group, 1994;
Shepherd et al., 1995). That level of
adher-ence/compliance could only be crudely indicated from these trials’
reliance on returned tablet/capsule counts as estimators of patients’ exposure
to the test drugs, for reasons discussed later. It remains to be seen how many
patients in big clinical trials continue to keep scheduled appointments but surreptitiously
discon-tinue dosing, or take too few doses to have more than de minimus
clinical effects. Suffice it to say, though, that these confirmatory trials
certainly demonstrate that it is possible to maintain nominal persistence with
trial medications at a very high level.
It
seems reasonable to infer that the administra-tive apparatus of big clinical
trials – the process of securing informed consent, multiple phone calls from
trial staff to patients, other reminders, all adding up to more than usual
professional attention paid to patients – serve to keep the vast majority of
patients engaged with the treatment process over long periods of time, with an
evident > 90% persistence through year 5 – a stark contrast with the much
lower persis-tence observed in studies carried out on routine medi-cal practice
(Jones et al., 1995; Catalan and LeLorier, 2000; Benner et al., 2002).
ARE EXECUTION AND PERSISTENCE IMPROVABLE?
The
pharmionics field is just at the beginning of systematic work along these
lines, with as yet few published studies, and even fewer studies of
satis-factory design and analysis. The best in this cate-gory is the recently
published, 392-patient, one-year study (Vrijens et al., 2005a), which has shown that community pharmacies,
cluster-randomized between practice-as-usual and measurement-guided
interven-tion, could use electronically compiled drug dosing histories to guide
their interventional discussions with the patients, and achieve a statistically
signif-icant improvement in both persistence and compli-ance with the daily
dosing regimen of atorvastatin, a leading drug in the statin class. This result
clearly needs to be repeated, and to benefit from knowl-edge of, and avoidance
of, problems that lurked beneath the surface of this study. For example, the
interventional programme was designed by commit-tee, several members of which
were adamant that the provision of a credit card-sized beeper would suffice to
remind patients when to take the once-daily dose; in the event, however, only
22% of patients accepted the beeper card, and half of those rapidly
discontinued its use – a phenomenon well known in the consumer electronics
arena as ‘beeper-fatigue’. Another limitation was that each pharmacist in the
intervention group was allowed to improvise his/her interventional manoeuvres.
Despite
these problems, however, the study showed clear-cut benefits of
measurement-guided medication management, as improvised on intuitive grounds by
community pharmacists. The results of this study are probably best seen as a
starting point for learning-curve-based improvements in results, combined with
simplifications in method and corre-sponding economies.
TAXONOMIC ISSUES AND THEIR RELATION TO SOUND ANALYSES OF DOSING HISTORY DATA
The
foregoing discussion makes clear the three major categories of deviation from a
prescribed drug dosing regimen in ambulatory care: acceptance, execution and
discontinuation. The time between the first-taken and the last-taken dose is
called ‘persistence’, expressed in units of time. The quality of regimen
execution is the outcome of a comparison between the patient’s dosing history
and the prescribed drug dosing regimen – the outcome of the comparison of two
time-series. As there are many facets to time-series data, there is no single
parameter that captures all facets, so there are a number of ways to express
the data.
Many
investigators have used only aggregate expressions such as the percentage of
prescribed doses taken, the percentage of days on which the correct number of
doses was taken, or the percentage of interdose intervals that fall within
certain limits of the interval implicit in the prescription, for exam-ple 24
hours for once-daily dosing. Aggregate figures across long periods of time hide
informative time-variations in dose-taking behaviour. For example, there is a
marked ‘weekend effect’ frequently evident, by which substantially and
significantly more doses are missed on weekends than on weekdays. Another
time-dependency is the tendency for the quality of regimen execution to decline
gradually over long peri-ods of time.
The
choice of limits on the dosing interval should ideally relate to the
pharmacometric properties of the drug in question, for example
bendroflumethazide, the diuretic widely used in the United Kingdom for
hyper-tension treatment, has a 3-hour plasma half-life (Jackson, 1995), but a
6.3-day duration of anti-hypertensive action after a last-taken dose; if one
considers only the pharmacokinetic properties of that drug, the range would be
set quite narrowly, perhaps ±1
5 hours, but given that the pharmacodynamic properties of the drug dominate,
and confer a 6.3-day duration of action (Girvin and Johnston, 2004), one could
reasonably accept a range of ±2
days.
In
the known pharmacometric properties of bendroflumethazide, one gets a glimpse
of how the search for a sound quantitative answer to the question ‘how much
adherence is enough’ represents a chal-lenge to pharmacometric understanding of
drugs and the dose- and time-dependencies of their actions. It also emphasizes
the importance of examining not only pharmacokinetic information about the drug
in ques-tion, but also pharmacodynamic information, particu-larly the duration
of drug action(s) after a last-taken dose. Either can be the determining factor
in judging ‘how much adherence is enough’, which of course is a crucial but
neglected aspect of determining an optimal drug dosing regimen. The ‘neglect’
arises probably in large part from the prevailing delusion that achieving a
once-daily dosing regimen for a product will auto-matically solve adherence
problems. The case studies presented later serve to disabuse anyone of that
naive notion.
Contrasting Dynamics of
Acceptance, Execution, Discontinuation: why no Single Parameter can Encompass
all Major Dosing Errors and Support Sound Quantitative Analysis
Acceptance
and discontinuation are more or less binary occurrences, in that they are
usually abrupt. Execution, in contrast, is a continuous process that can vary
within days, between days, from week to week, or from month to month, and
indeed does so, as noted above. It is not possible to combine binary and
continuous processes in one parameter, except in a literary sense, but
certainly not in the sense of having one parameter that supports sound,
quantitative analysis.
‘Adherence’
is generally used as a blanket term for all aspects of how well or poorly a
prescribed drug dosing regimen is followed by patients. As a literary
expression, it serves a certain purpose, but it does not support sound
measurement, which must distinguish between non-acceptance, poor execution and
early discontinuation. As a concrete example, consider the following statement
by the 6th Joint National Commission on High Blood Pressure (The Sixth Report
of the Joint National Committee on Prevention, Detection, Evaluation, and
Treatment of High Blood Pressure, 1997): ‘Poor adherence to anti-hypertensive
therapy remains a major therapeu-tic challenge, contributing to the lack of
adequate control in more than two-thirds of patients with hypertension.’ The
problem with this statement is that it does not distinguish between
non-acceptance, poor execution or early discontinuation. In all likelihood each
plays some role in the overall problem. Based on Figure 48.1, which includes a
considerable amount of data from studies of hypertensive patients, early
discontinuation is almost certainly the biggest contributor to the distressing
shortfall in the quality of anti-hypertensive drug treatment. As the Belgian
atorvastatin study illus-trates, a programme of measurement-guided medi-cation
management can not only prolong patients’ engagement with the drug dosing
regimen, other-wise known as extended persistence, but if we are to do better
than the results in that study, it prob-ably means attacking specifically each
of the three major errors: non-acceptance, poor execution, early
discontinuation.
The Importance of Distinguishing Early Discontinuation and Poor Execution in Analysing Drug Dosing History Data
A
common manner of expressing adherence/ compliance data goes as follows:
Rates
of adherence for individual patients are usually reported as the percentage of
the prescribed doses of the medication actually taken by the patient over a
specified period The average rates of adherence in clinical trials can be remarkably
high, owing to the attention study patients receive and to selection of the
patients, yet even clinical trials report average adher-ence rates of only 43
to 78 percent among patients receiving treatment for chronic conditions
(Osterberg and Blaschke, 2005).
Expressing
the percentage of prescribed doses taken during a fixed interval of time
inevitably mixes together execution and early discontinuation. Thus, a patient
will be categorized as having 50% adher-ence who doses strictly punctually but
discontinues at month 6 in a 12-month study. Of course, if dura-tion of the
study had been set at 24 months, then the patient who discontinues at 6 months
would be cate-gorized as a 25% adherer. Also categorized as a 50% adherer will
be a patient whose execution is such that he takes only half the prescribed
doses, but continues to be engaged with the dosing regimen throughout the
12-month study. These two contrasting patterns of dosing, both of which are
common occurrences, not exotic oddities, call for very different interventional
approaches: targeted motivation in the first patient to abort his intention to
quit, versus a careful review with the patient of his day-to-day dosing
patterns, with assistance in finding robust routines in his daily life to which
his daily dosing can be linked, as suggested by Cramer and Rosenheck (Cramer
and Rosenheck, 1999). Then ongoing follow-up is needed in the latter instance
to see how well specific suggestions work and to provide changes and/or
motivation, as needed, to maintain high quality of execution. Ongoing
obser-vation of daily dosing patterns may, if the quality of execution starts
to dwindle, signal a pending episode of discontinuation.
There
are two important points in the foregoing. One is that the improvement of poor
quality of execu-tion is self-evidently a more difficult management problem
than is the postponement of discontinuation to achieve longer persistence. The
second point is that it is a fundamental mistake in the analysis of dosing
history data to ignore the distinction between poor execution and short
persistence. ‘Execution’ self-evidently relates to what happens while the
patient is engaged with the dosing regimen; when that engage-ment halts,
execution is finished.
One
might argue that, from a practical point of view, taking half the prescribed
doses is the same, whether it occurs by ongoing faulty execution or by early
discon-tinuation of correct execution. The counter-arguments are as follows.
First, since there appears to be a major difference in the complexity and cost
of interven-ing to improve execution vs. intervening to prolong persistence, we
only engage with intervention when we know which we are trying to fix. Second,
life goes on past the end of an arbitrarily defined study period, so that the
patient who has quit taking the medicine will, unless re-recruited, generate no
revenues for the manufacturer/developer/marketer, whereas the faulty executor
will, for as long as he persists, continue to generate revenues, albeit at a
rate reduced by the extent of his ongoing underdosing. Third, the percent-age
of prescribed doses taken by the short persis-ter varies with the duration of
the study, as noted above; in contrast, the percentage of prescribed doses
taken by a consistently poor executer is unchanged by altering the duration of
the study, setting aside the tendency for the quality of execution to decline
gradually with time since the start of treatment.
Figure
48.1 provides the best format for express-ing the basic findings from analysis
of drug dosing histories in groups of patients. One can and should go further
to characterize the occurrence of omitted doses and drug holidays on a
patient-by-patient basis. The clinical correlates of substantial underdosing
should be examined carefully, as they may, among other things, show whether the
recommended drug dosing regimen provides either insufficient or a substantial
excess of ‘forgiveness’, which is defined as the post-dose duration of the
drug’s therapeutically effective action(s) minus the recommended drug dosing
interval (Urquhart, 1997).
Note
that Figure 48.1 is a very simple, straight-forward summary of pharmionic data.
Complexity in this field arises at the level of individual pharma-ceuticals,
because each has its own recommended dosing regimen and pharmacokinetic and
pharmaco-dynamic properties. The clinical and economic conse-quences of early
discontinuation, dose omissions, and drug holidays will depend directly on
these product-specific properties. They are indeed more than drug-specific,
because differences in drug formulation can not only prolong drug entry into
the bloodstream, but also alter its rate in sometimes clinically impor-tant
ways – a key example being how the pharmaco-dynamics of nifedipine were
beneficially altered by its re-formulation in an oral, osmotic pump dosage form
that releases the drug at a constant rate, versus the rapid highly time-varying
release profile asso-ciated with the original dosage form (Breimer and
Urquhart, 1993). Thus, the main complexities in this field arise from the fact
that each of hundreds of phar-maceutical products can be expected to have
different answers to the question of the clinical and economic consequences of
commonly occurring dosing errors.
THE SPECIAL ROLE OF DRUG HOLIDAYS
The
usually abrupt cessation and resumption of dosing that characterizes drug
holidays provide an opportu-nity to search for important clinical correlates
that may contribute to the understanding of adverse reac-tions occasioned by
either rebound effects, as dosing stops, or recurrent first-dose effects when
post-holiday dosing resumes in patients who should be re-titrated after some
period of interrupted dosing, as was done prior to the initial start of
treatment. One of the missing elements of pharmacodynamic information about
drugs with first-dose effects is the length of time, after dosing stops, needed
to restore drug naiveté and the need for re-titration for least-hazardous
resumption of dosing post-holiday. Such information would inform the answering
of reasonable questions about the role of drug holidays and their potential
hazards in trials of drugs like, for example, encainide and flecainide, which
have hazardous or even lethal pro-arrhythmic effects that are triggered by
unduly high rates of dose-escalation in the drug-naïve state. By the same
token, the role of drug holidays remains unclear in the case of peripheral
vasodilators that can have hazardous hypotensive episodes or reflex tachycardia
when the rate of dose-escalation is too high, or full-strength dosing resumes
abruptly in the drug-naïve state.
While
the various patterns and extents of underdosing seen in patients’ dosing
histories are, in a strict sense, observational data, their clinical correlates
may send up useful ‘red flags’, tentatively identifying, for example, dosing
regimens that are set too high (Cross et
al., 2002; Heerdink, Urquhart and Leufkens, 2002), hazardous rebound effects (Urquhart, 1997) and recur-rent
first-dose effects (Urquhart, 1997). Clinical corre-lates of a single holiday
would naturally be difficult to interpret, but if holidays recur, as they do in
some patients, one has the potential opportunity to see repe-tition of holidays
and their associated events. Repeti-tion and consistent time-sequence greatly
strengthen the inference of causality. A common problem, of course, is that
most clinical events cannot be measured continuously, and are only
intermittently sampled, which, via white-coat compliance (Feinstein, 1990), is
likely to prevent the occurrence of holidays in temporal proximity to the
sampled clinical events. In contrast, holidays can be captured by means of
automatic, continuous electronic compilation of drug dosing histories.
A
noteworthy technical advance is the ability of the latest generation of
implanted cardiac pacemakers and defibrillators to automatically compile
complete records of electrophysiological activity through-out multi-week
intervals between data-downloads. That capability, combined with the prevalence
of pro-arrhythmic effects among leading cardiac anti-arrhythmic drugs, provides
a potentially rich area for enlightening research on the pharmacodynamics of
the anti-arrhythmic drugs.
Related Topics
