Cardiovascular Safety Signal Evaluation
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: NSAIDs - COX-2 Inhibitors – Risks and Benefits
These data are avail-able from the FDA website (FDA, 2001) and some were subsequently published in peer-reviewed journals.
CARDIOVASCULAR SAFETY SIGNAL
EVALUATION
As
results from VIGOR indicated that rofecoxib was associated with increased risk
of myocardial infarction, a Food and Drug Administration (FDA) advisory
committee meeting was convened in February 2001 to review gastrointestinal and
cardiovascular safety data of rofecoxib and celecoxib. These data are
avail-able from the FDA website (FDA, 2001) and some were subsequently
published in peer-reviewed journals. In the US, revised package insert of
rofecoxib with updated safety information was released in April 2002 (Kweder,
2004).
BIOLOGICAL MECHANISM
As
the COX-2 inhibitors have minimal effects on COX-1 in gastric epithelium, they
have more favor-able gastrointestinal safety profile than the non-selective
NSAIDs. However, at the time of approval of celecoxib and rofecoxib,
pharmacologic stud-ies indicated that selective inhibition of COX-2 may
contribute to imbalance in the prostacyclin (prostaglandin I2) to
thromboxane ratio and result in thrombotic events (FitzGerald, 2004). COX-2 in
endothelium is responsible for the production of prostacyclin, which inhibits
platelet aggregation, causes vasodilatation, and prevents proliferation of
vascular smooth-muscle cells. On the other hand, the COX-1 in platelets, which
is not affected by the COX-2 inhibitors, is responsible for thromboxane A2
synthesis, and thromboxane A2 has the opposite effects of
prostacyclin, causing platelet aggregation, vasoconstriction, and vascular
proliferation. Theoret-ically, selective inhibition of COX-2 would allow the
physiologic effects of thromboxane to predomi-nate and result in adverse
cardiovascular outcomes (FitzGerald, 2003).
The
VIGOR investigators cited a study in healthy volunteers that showed different
effects on thrombox-ane A2 production and platelet aggregation
associated with different non-selective NSAIDs (Van Hecken et al., 2000). Ibuprofen 800 mg three times a day and sodium naproxen 550 mg twice a day in
healthy volun-teers showed substantial inhibition effects on throm-boxane A2
production and platelet aggregation, but how these pharmacologic findings
translate to preven-tion of myocardial infarction by naproxen needed to be
verified in clinical studies. The cardio-protective effects of naproxen would
have to be very power-ful in order to result in the VIGOR findings and support
the hypothesis that rofecoxib 50 mg per day was not associated with increased
risk of myocardial infarction.
CLINICAL TRIALS OF CELECOXIB AND ROFECOXIB
After
the results of clinical trials discussed during the February 2001 FDA advisory
committee meet-ing were made public, a group of independent inves-tigators
reviewed cardiovascular events observed in CLASS, VIGOR, and two unpublished
clinical trials of rofecoxib (Mukherjee, Nissen and Topol, 2001). The review
covered more cardiovascular data than that was published in the original CLASS
and VIGOR reports in 2000 as patients contributed more follow-up time and
cardiovascular events were independently adjudicated. Reviews of cardiovascular
safety data in the clinical development programs of celecoxib and rofecixb were
also published by manufacturers of the drugs from 2001 through 2003.
Celecoxib
No
increased risk of myocardial infarction, stroke, or death was found in the
celecoxib group in CLASS. The same finding was observed in patients who
received aspirin and those who did not receive aspirin during the trial
(Mukherjee, Nissen and Topol, 2001). The CLASS investigators carried out a
safety analy-sis and evaluated the risk of cardiovascular outcomes,
cerebrovascular outcomes, and peripheral vascular outcomes (White et al., 2002). No increased risk of
thromboembolic events was found among the cele-coxib group (Table 47.1). White
and colleagues combined data from 15 trials of celecoxib and used the
Anti-Platelet Trialists’ Collaboration (APTC) defini-tion of adverse
cardiovascular outcomes (Antiplatelet Trialists’ Collaboration, 1994),
including cardiovas-cular, hemorrhagic and unknown death, myocardial
infarction, and cerebrovascular accident, as the end point of interest (White et al., 2003). A relative risk of 1.06
(95% CI, 0.70–1.61) was found for the celecoxib (all doses) versus
non-selective NSAID comparison.
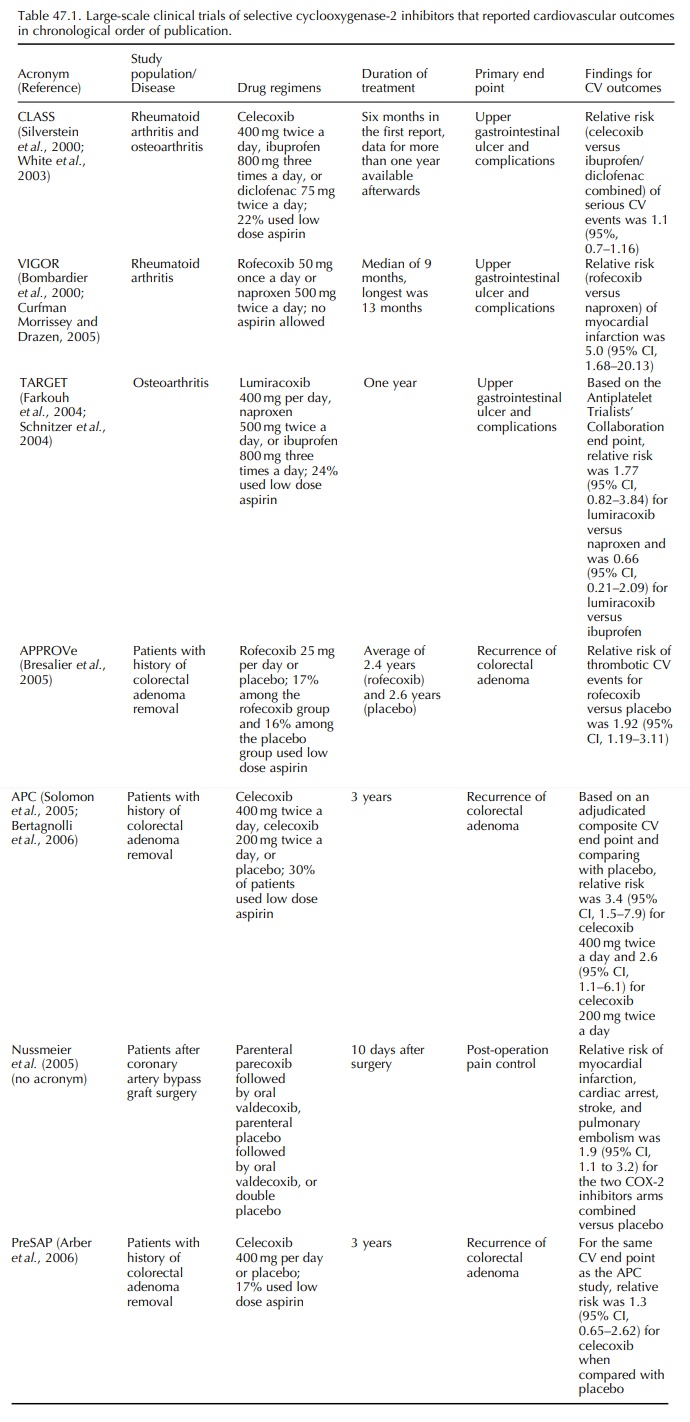
Rofecoxib
Two
unpublished studies, 085-2001 and 090-2001, of rofecoxib reported by Mukherjee
and colleagues in 2001 were three-arm trials of rofecoxib 12.5 mg per day,
nabumetone 1000 mg per day, and placebo for six weeks among patients with
osteoarthritis. Using low dose aspirin was not an exclusion criterion in both
trials. No difference in cardiovascular events was found between the rofecoxib-
and nabumetone-treated groups in 085-2001 and there was a non-significant
increase in the incidence of cardiovascular events in the rofecoxib versus
nabumetone comparison (1.5% versus, 0.5%) in 090-2001.
For
VIGOR, Mukherjee and colleagues reported incidence of serious thrombotic
cardiovascular events, including myocardial infarction, unstable angina,
cardiac thrombus, resuscitated cardiac arrest, sudden or unexplained death,
ischemic stroke, and transient ischemic attacks in the treatment groups
(Mukherjee, Nissen and Topol, 2001). In addition, more adju-dicated cases of
serious cardiovascular events were reported at the February 2001 FDA advisory
commit-tee, which did not appear in the original VIGOR publication (Curfman,
Morrissey and Drazen, 2005). The VIGOR study protocol stated that history of
cere-brovascular events in the two years before the study, history of
myocardial infarction or coronary bypass in the year before the study, and
requirement for or ongo-ing treatment with aspirin, ticlopidine, or
anticoagu-lants were exclusion criteria. However, ‘requirement for aspirin
treatment’ was not objectively defined and was subjectively assessed by the
physicians who enrolled the patients. Mukherjee and colleagues reported a post-hoc
stratification of study subjects according to cardiovascular indication for
aspirin use, which was operationally defined as prior history of stroke,
transient ischemic attack, myocardial infarc-tion, unstable angina, angina
pectoris, coronary artery bypass graft surgery, or percutaneous coronary
inter-ventions. Only 321 (4%) of the 8076 enrolled subjects were
aspirin-indicated and no myocardial infarction occurred among aspirin indicated
patients. Relative risk of serious cardiovascular events was 4.89 (95% CI,
1.41–16.88) among the rofecoxib (50 mg per day) group in the full cohorts and
for the non-aspirin indi-cated subjects, the relative risk was 1.89 (95% CI,
1.03–3.45).
Scientists
from the manufacturer of rofecoxib and academic investigators analyzed the
safety database of clinical trials of rofecoxib and published three safety
reports after VIGOR. In the first report, Konstam and colleagues reviewed
combined data of more than 28 000 patients from 23 trials and used the APTC
defi-nition for cardiovascular end point (Konstam et al., 2001). Rofecoxib was associated with increased risk of APTC
events when compared with naproxen (rela-tive risk, 1.69, 95% CI, 1.07–2.69)
but there was no increased risk when rofecoxib was compared with placebo or
non-naproxen NSAIDs. Stratifying the rofecoxib group by dose and compared with
all NSAIDs, relative risk was 2.08 (95% CI, 0.57, 7.51) for rofecoxib 50 mg per
day and was 1.16 (95% CI, 0.25, 7.18) for rofecoxib 25 mg per day. Reicin and
colleagues reported results from pooled data from eight phase IIB or III trials
of rofecoxib, ibuprofen, diclofenac, nabumetone, or placebo for
osteoarthri-tis, with a total of 5435 patients (Reicin et al., 2002). Using the APTC end point and any arterial or venous
thrombotic cardiovascular adverse event as the outcome of interest, no
significantly increased cardiovascular risk was observed in the rofecoxib–
non-selective-NSAID and rofecoxib–placebo compar-isons. However, statistical
power was limited to detect a modest increase in cardiovascular risk among a
dataset of this size and these trials were short-term studies with no
information on potential effects among long-term use. In 2003, Weir and
colleagues reported safety data from the rofecoxib development program that
included short-term trials, long-term studies like VIGOR, and
placebo-controlled studies for the prevention of Alzheimer’s disease (Weir et al., 2003). The results confirmed the
increased cardio-vascular risk for the rofecoxib–naproxen comparison, with a
relative risk of 1.61 (95% CI, 1.04–2.50) in pooled analysis for trials among
patients with rheuma-toid arthritis and/or osteoarthritis. No increased
cardiovascular risk was found for the rofecoxib– non-naproxen-NSAID comparison
or the rofecoxib– placebo comparison. These data pooling exercises were helpful
in the safety assessment of drugs, but the heterogeneity of the study
populations and treat-ment periods are major limitations. In the report by Weir
and colleagues, there were no stratified results based on dose and duration of
use for rofecoxib.
CARDIOPROTECTIVE EFFECTS OF NAPROXEN IN EPIDEMIOLOGY STUDIES
While
there has been pharmacologic basis for cardio-protective effects of naproxen
(Van Hecken et al., 2000), there has
been no reported clinical trial that specifically evaluated the risk of adverse
cardiovas-cular outcomes in a naproxen versus placebo compar-ison before VIGOR
or through 2005. The VIGOR results prompted more than 10 reports of
observa-tional studies that evaluated the association between naproxen use and
myocardial infarction, all of them were included in a meta-analysis conducted
by Jüni and colleagues (Jüni et al.,
2004). Reduced risk of myocardial infarction associated with naproxen was
reported in three case-control studies; reduced but non-significant risk was
reported in four studies; and small increased risk associated with naproxen was
reported in three studies. All these data taken together suggested a small
reduced risk, on the order of 15%, of myocardial infarction associated with
naproxen use. However, these findings did not support the hypothesis that
rofecoxib 50 mg per day did not increase risk of myocardial infarc-tion, as the
small reduction in risk associated with naproxen use could not adequately
explain the more than fourfold increase in risk of myocardial infarc-tion in
the rofecoxib–naproxen comparison observed in VIGOR.
OBSERVATIONAL STUDIES OF CELECOXIB AND ROFECOXIB
After
VIGOR, important information about population-based cardiovascular risk
associated with rofecoxib and celecoxib came from large observa-tional studies
with sufficient numbers of COX-2 inhibitors users in a real life setting. Ray
and colleagues reported that new users of high dose rofecoxib (more than 25 mg
per day) had an almost twofold increase in the risk of hospital admission for
acute myocardial infarction or death from coronary heart disease when compared
with those who did not receive any non-selective NSAID or COX-2 inhibitor (Ray et al., 2002; Table 47.2). No
statistically significant increased risk was observed among new users of lower
dose rofecoxib (25 mg or less per day), celecoxib, naproxen, or ibuprofen.
Three
other observational studies, one each from Canada, the UK, and the US, were
published before the market withdrawal of rofecoxib in September 2004 and are
summarized in Table 47.2. Mamdani compared new users of COX-2 inhibitors or
non-selective NSAIDs with subjects who did not use any COX-2 inhibitors or
non-selective NSAID and reported no increased risk in acute myocardial
infarc-tion in new users of celecoxib or rofecoxib (Mamdani et al., 2003).
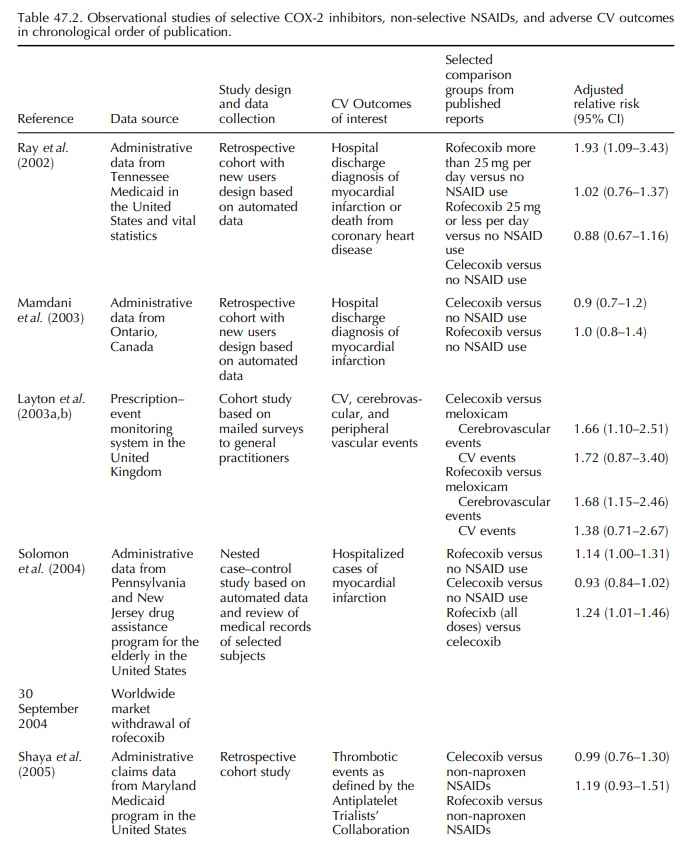
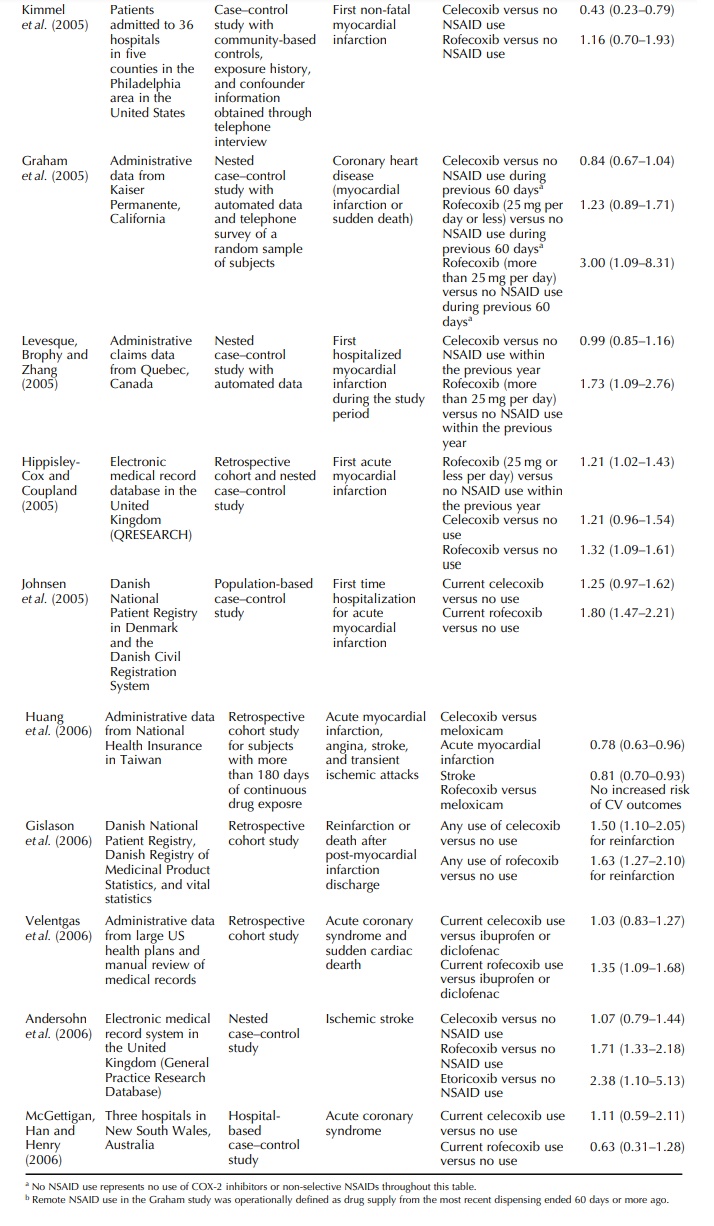
The
UK study was based on the Prescription-Event Monitoring system and was reported
as two companion articles in the same journal in 2003 – one was a comparison
between celecoxib and meloxicam (Layton et
al., 2003a) and the other was a compari-son between rofecoxib and meloxicam
(Layton et al., 2003b). Outcomes of
interest were cardiovascular, cerebrovascular, and peripheral venous thrombotic
events. Comparing with meloxicam, a preferential but not selective COX-2
inhibitor, and only adjusted for age and sex, celecoxib and rofecoxib were both
associated with increased risk of cerebrovascular events. Age-sex-adjusted
relative risk for cardiovas-cular events suggested an increased risk for both
drugs but the 95% confidence intervals for both relative risks included one.
The
manufacturer of rofecoxib funded a case-control study among members of
state-sponsored pharmacy benefit programs for residents aged 65 or older of New
Jersey and Pennsylvania in the US (Solomon et
al., 2004). Comparing rofecoxib with no use of non-selective NSAIDs or
COX-2 inhibitors, naproxen, ibuprofen, or other NSAIDs, relative risk estimates
for developing acute myocardial infarction were 1.14, 0.95, 1.21, and 1.17
respectively. Compar-ing celecoxib against the same drug groups, the relative
risk estimates ranged between 0.93 and 0.98. 95% CIs for these eight relative
risks all included one. Consis-tently higher relative risk of acute myocardial
infarc-tion was associated with the use of more than 25 mg per day of rofecoxib
than that for the use of 25 mg per day or less of rofecoxib when compared with
the same groups. No dose-dependent finding was observed for celecoxib.
Rofecoxib was associated with a 24% increased risk of hospitalized myocardial
infarction when compared with celecoxib (Table 47.2).
NON-THROMBOEMBOLIC ADVERSE CARDIOVASCULAR EVENTS
Other
adverse cardiovascular effects of COX-2 inhibitors have also been reported
after their approval. Whelton and colleagues conducted a 6-week clin-ical trial
of COX-2 inhibitors among patients 65 years or older with osteoarthritis and
stable medication-controlled hypertension and showed that the incidence of
increased systolic blood pressure was higher among patients randomly assigned
to receive 25 mg of rofecoxib per day than among those who received celecoxib
200 mg per day (Whelton et al.,
2002). Using automated administrative data from Ontario, Canada, Mamdani and
colleagues reported an increased risk of congestive heart failure among
rofe-coxib users but not celecoxib users (Mamdani et al., 2004).
SUMMARY OF SAFETY INFORMATION OF CELECOXIB AND ROFECOXIB THROUGH JULY 2004
Results
from VIGOR clearly indicated that rofecoxib 50 mg per day was associated with
higher risk of adverse cardiovascular thrombotic events. Epidemiol-ogy studies
reported by Ray and colleagues in 2002 and by Solomon and colleagues in 2004
corrobo-rated this finding. Whether the use of rofecoxib 25 mg or less per day
increased the risk of serious cardio-vascular thrombotic events was less
certain. On the other hand, no compelling evidence suggested that celecoxib use
was associated with increased risk of adverse cardiovascular outcomes and as of
mid-2004 these results did not support a class effect of adverse cardiovascular
effects for the COX-2 inhibitors. In the label revision for rofecoxib in 2002,
increased cardiovascular risk observed from the VIGOR trial was noted and
recommendation that high dose rofe-coxib not to be used chronically was added
(Kweder, 2004).
Related Topics
