Dressings
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Sterile Pharmaceutical Products
Dressings and surgical materials are used widely in medicine, both as a means of protecting and providing comfort for wounds and for many associated activities such as cleaning and swabbing. They may or may not be used on areas of broken skin.
DRESSINGS
Dressings and surgical materials are
used widely in medicine, both as a means of protecting and providing comfort
for wounds and for many associated activities such as cleaning and swabbing.
They may or may not be used on areas of broken skin. If there is a potential
danger of infection arising from the use of a dressing then it must be sterile.
For instance, sterile dressings must be used on all open wounds, both surgical
and traumatic, on burns, and during and after catheterization at a site of
injection. It is also important to appreciate that sterile dressings must be
packaged in such a way that they can be applied to the wound aseptically.
Dressings are described in the British Pharmacopoeia (2010). Methods for their
sterilization include autoclaving, dry heat, ethylene oxide and ionizing
radiation. Any other effective method may be used. The choice is governed
principally by the stability of the dressing constituents to the stress applied
and the nature of their components. Most celluloses and synthetic fibres
withstand autoclaving, but there are exceptions. For instance, boric acid
tenderizes cellulose fibres during autoclaving, and dressings containing waxes
cannot be sterilized by moist heat. Certain constituents are also adversely
affected on exposure to large doses of gamma radiation. Examples of dressings
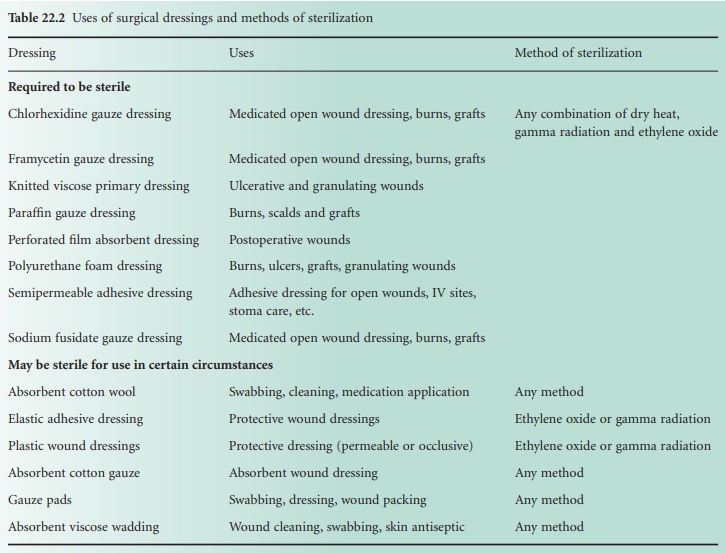
that are required to be sterile are listed in Table 22.2,
together with other dressings and materials that may be sterilized when
required.
A very important aspect of the production of dressings
is packaging. The packaging material must allow correct sterilization conditions (e.g.
permeation of moisture
or ethylene oxide), retain
the dressing in a sterile
condition and allow for its removal
without contamination prior
to use. All dressings intended
for aseptic handling
and application must be double
wrapped. For steam
sterilization they may be individually wrapped in fabric,
paper or nylon and sterilized in metal drums,
cardboard boxes or bleached Kraft paper. The choice of method also determines the design of the autoclave cycle. Providing that adequate steam penetration is assured, dressings may be sterilized in downward
displacement autoclaves which rely on displacement of air by steam. However, high
prevacuum autoclaves in which virtually all the air is removed before the admission of steam are much more commonly
employed. This method
ensures rapid heating
up of dressings, reduces the time needed
to achieve sterilization (e.g. 134 °C for 4 minutes) and shortens the overall sterilization cycle.
A recent
development is the use of spray-on dressings. A convenient type is an acrylic polymer
dissolved in ethyl acetate and packed as an aerosol.
This should be self-sterilizing. The
film after application is able to maintain the sterility of a clean wound for up to 2 weeks. However, they can only be used on clean,
relatively dry wounds.
Related Topics
