Evaluation of Liquid Disinfectants
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Laboratory Evaluation Of Antimicrobial Agents
Phenol coefficient tests were developed in the early 20th century when typhoid fever was a significant public health problem and phenolics were used to disinfect contaminated utensils and other inanimate objects.
EVALUATION OF LIQUID DISINFECTANTS
a)
General
Phenol coefficient tests were developed in the early 20th century when
typhoid fever was a significant public health problem and phenolics were used
to disinfect contaminated utensils and other inanimate objects. Details of such
tests can be found in earlier editions of this book. However, as non-phenolic
disinfectants became more widely available, tests that more closely paralleled
the conditions under which disinfectants were being used (e.g. blood spills)
and which included a more diverse range of microbial types (e.g. viruses,
bacteria, fungi, protozoa) were developed. Evaluation of a disinfectant’s
efficacy was based on its ability to kill microbes, i.e. its cidal activity,
under environmental conditions mimicking as closely as possible real life situations.
As an essential component of each test was a final viability assay, removal or
neutralization of any residual disinfectant (to prevent ‘carryover’ toxicity)
became a significant consideration.
The development of methods to evaluate
disinfectant activity in diverse environmental conditions and to determine
suitable in-use concentrations/dilutions to be used led to the development by
Kelsey, Sykes and Maurer of the so-called capacity-use dilution test which
measured the ability of a disinfectant at appropriate concentrations to kill
successive additions of a bacterial culture. Results were reported simply as
pass or fail and not a numerical coefficient. Tests employed disinfectants
diluted in hard water (clean conditions) and in hard water containing organic
material (yeast suspension to simulate dirty conditions), with the final
recovery broth containing 3% Tween 80 as a neutralizer. Such tests are
applicable for use with a wide variety of disinfectants (see Kelsey &
Maurer, 1974). Capacity tests mimic the practical situations of housekeeping
and instrument disinfection, where surfaces are contaminated, exposed to
disinfectant, re contaminated and so forth. The British Standard (BS 6907:1987)
method for estimation of disinfectants used in dirty conditions in hospitals by
a modification of the original Kelsey-Sykes test is the most widely employed
capacity test in the UK and Europe. In the USA, effectiveness test data for
submission must be obtained by methods accepted by the Association of Official
Analytical Chemists, known collectively as Disinfectant Effectiveness Tests
(DETs).
However, the best information
concerning the fate of microbes exposed to a disinfectant is obtainable by
counting the number of viable cells remaining after exposure of a standard
suspension of cells to the disinfectant at known concentration for a given time
interval— suspension tests. Viable counting is a facile technique used in many
branches of pure and applied microbiology. Assessment of the number of viable
microbes remaining (survivors) after exposure allows the killing or cidal
activity of the disinfectant to be expressed in a variety of ways, e.g.
percentage kill (e.g. 99.999%), as a log10 reduction
in numbers (e.g. 5-log killing), or by log10 survival
expressed as a percentage. Examples of such outcomes are shown in Table 18.1.
Unfortunately, standardization of the
methodology to be employed in these efficacy tests has proven difficult, if not
impossible, to obtain, as has consensus on what level of killing represents a
satisfactory and/or acceptable result. It must be stressed, however, that
unlike tests involving chemotherapeutic agents where the major aim is to
establish antimicrobial concentrations that inhibit growth (i.e. MICs),
disinfectant tests require determinations of appropriate cidal levels. Levels
of killing required over a given time interval tend to vary depending on the
regulatory authority concerned. While a 5-log killing of bacteria (starting
with 106 CFU/ml) has been suggested for suspension
tests, some authorities require a 6-log killing in simulated use tests. With
viruses, a 4-log killing tends to be an acceptable result, while with prions it
has been recommended that a titre loss of 104 prions
should be regarded as an indication of appropriate disinfection provided that
there has been adequate prior cleaning. With simulated use tests, cleaning
followed by appropriate disinfection should result in a prion titre loss of at
least 107.
b) Antibacterial Disinfectant
Efficacy Tests
Various regulatory authorities in Europe (e.g. European Standard or
Norm, EN; British Standards, BS; Germany, DGHM; France, AFNOR) and North
America (e.g. Food and Drug Administration, FDA; Environmental Protection
Authority, EPA; Association of Official Analytical Chemists, AOAC) have been
associated with attempts to produce some form of harmonization of dis-infectant
tests. Perhaps the most readily accessible and recent guide to the methodology
of possible bactericidal, tuberculocidal, fungicidal and viricidal disinfectant
efficacy tests, is that of Kampf and colleagues (2002). This publication
summarizes and provides references to various EN procedures (e.g. prEN 12054).
i) Suspension tests
While varying to some degree in their
methodology, most of the proposed procedures tend to employ a standard
suspension of the microorganism in hard water containing albumin (dirty
conditions) and appropriate dilutions of the disinfectant—so-called suspension
tests. Tests are carried out at a set temperature (usually around room
temperature or 20°C), and at a selected time interval samples are removed and
viable counts are performed following neutralization of any disinfectant
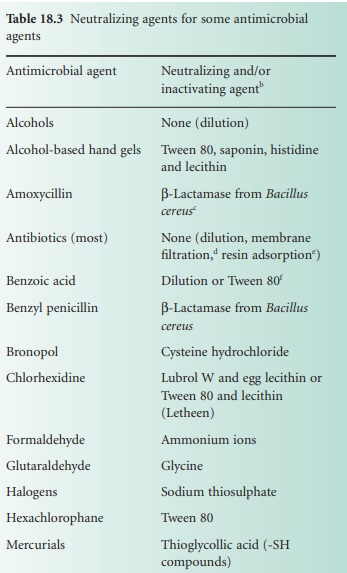
remaining in the sample. Neutralization or inactivation of residual
dis-infectant can be carried out by dilution, or by addition of specific agents
(see Table 18.3).
Using viable counts, it is possible to calculate the concentration of disinfectant
required to kill 99.999% (5-log kill) of the original sus-pension. Thus 10 survivors
from an original population of 106 cells represents a 99.999% or 5-log kill. As
bacteria may initially decline in numbers in diluents devoid of additional
disinfectant, results from tests incorporating disinfectant-treated cells can
be compared with results from simultaneous tests involving a
non-disinfectant-containing system (untreated cells). The bactericidal
effect BE can then be
expressed as:

·
Other than dilution.
D/E neutralizing media—adequate for QACs, phenols, iodine and chlorine
compounds, mecurials, formaldehyde and glutaraldehyde (see Rutala, 1999).
·
O ther appropriate enzymes can be
considered, e.g. inactivating or modifying enzymes for chloramphenicol and aminoglycosides,
respectively.
·
F ilter microorganisms on to membrane,
wash, transfer membrane to growth medium.
·
R esins for the absorption of
antibiotics from fluids are available.
·
Tween 80 (polysorbate 80).

where NC and ND represent the final number of CFU/ml remaining
in the control and disinfectant series, respectively.
Unfortunately, viable count procedures are based on the assumption that
one colony develops from one viable cell or one CFU. Such techniques are,
therefore, not ideal for disinfectants (e.g. QACs such as cetrimide) that
promote clumping in bacterial suspensions, although the latter problem may be
overcome by adding nonionic surface active agents to the diluting fluid.
ii) In-use and simulated use
tests
Apart from suspension tests, in-use testing of used medical devices, and
simulated use tests involving instruments or surfaces deliberately contaminated
with an organic load and the appropriate test microorganism have been
incorporated into disinfectant testing protocols. An example is the in-use test
first reported by Maurer in 1972. It is used to determine whether the
disinfectant in jars, buckets or other containers in which potentially
contaminated material (e.g. lavatory brushes, mops) has been placed contain
living microorganisms, and in what numbers. A small volume of fluid is
withdrawn from the in-use container, neutralized in a large volume of a
suitable diluent, and viable counts are performed on the resulting suspension.
Two plates are involved in viable count investigations, one of which is
incubated for 3 days at 32°C (rather than 37°C, as bacteria damaged by
disinfectants recover more rapidly at lowered temperatures), and the other for
7 days at room temperature. Growth of one or two colonies per plate can be
ignored (a disinfectant is not usually a sterilant), but 10 or more colonies
would suggest poor and unsatisfactory cidal action.
Simulated use tests involve deliberate contamination of instruments,
inanimate surfaces, or even skin surfaces, with a microbial suspension. This
may either be under clean conditions or may utilize a diluent containing
organic material (e.g. albumin) to simulate dirty conditions. After being left
to dry, the contaminated surface is exposed to the test disinfectant for an
appropriate time interval. The microbes are then removed (e.g. by rubbing with
a sterile swab), resuspended in suitable neutralizing medium, and assessed for
viability as for suspension tests. New products are often compared with a known
comparator compound (e.g. 1 minute application of 60% v/v 2-propanol for hand
disinfection products—see EN1500) to show increased efficacy of the novel
product.
iii) Problematic bacteria
Mycobacteria are hydrophobic in nature
and, as a result, exhibit an increased tendency to clump or aggregate in
aqueous media. It may be difficult, therefore, to prepare homogeneous
suspensions devoid of undue cell clumping (which may contribute to their
resistance to chemical disinfection). As Mycobacterium tuberculosis is
very slow growing, more rapidly growing species such as M. terrae, M. bovis or M. smegatis can be substituted in tests (as
representative of M. tuberculosis). Recent global
public health concerns regarding the increasing incidences of tuberculosis
(including co-infections with HIV) in devel-oping, middletier and industrialized
nations brings into sharp focus the necessity for representative evaluations of
agents with potential tuberculocidal activity. This is particularly true given
the high proportion of cases classified as multidrug resistant tuberculosis
(MDR-TB). Apart from vegetative bacterial cells, bacterial or fungal spores can
also be used as the inoculum in tests. In such cases, incubation of plates for
the final viability determination should be continued for several days to allow
for germination and growth.
Compared with suspended (planktonic)
cells, bacteria on surfaces as biofilms are invariably phenotypically more
tolerant to antimicrobial agents. With biofilms, suspension tests can be
modified to involve biofilms produced on small pieces of an appropriate glass,
metal or polymeric substrate, or on the bottom of microtitre tray wells. After
being immersed in, or exposed to, the disinfectant solution for the appropriate
time interval, the cells from the biofilm are removed, e.g. by sonication, and
resuspended in a suitable neutralizing medium. Viable counts are then performed
on the resulting planktonic cells. Reduction in biomass following antimicrobial
challenge can be monitored using a standard crystal violet staining technique,
however, viable counting permits evaluation of rate of kill. The Calgary
Biofilm Device, permits the high-throughput screening of antimicrobial agents
against biofilms grown on 96 polycarbonate pegs in a 96-well microtitre plate.
Some important environmental bacteria survive in nature as intracellular
parasites of other microbes, e.g. Legionella pneumophila within
the protozoan Acanthamoeba polyphaga. Biocide
activity is significantly reduced against intracelluar legionellae (see Figure 18.3).
Disinfectant tests involving such bacteria should therefore be conducted both
on planktonic bacteria and on suspensions involving amoebae-containing
bacteria. With the latter, the final bacterial viable counts are performed
after suitable lysis of the protozoan host. The legionella/protozoa situation
may also be further complicated by the fact that the microbes often occur as
biofilms.
c) Other Microbe Disinfectant
Test
Suspension-type efficacy tests can also be performed on other microbes,
e.g. fungi, viruses, using similar techniques to that described above for
bacteria, although significant differences obviously occur in parts of the
tests.
i)
Antifungal (fungicidal) test
In order for disinfectants to claim
fungicidal activity, or for the discovery of novel fungicidal activities, a
range of standard tests have been devised. Perhaps the main problem with fungi
concerns the question of which morphological form of fungi to use as the
inoculum. Unicellular yeasts can be treated as for bacteria, but whether to use
spores (which may be more resistant than the vegetative mycelium) or pieces of
hyphae with the filamentous moulds, has yet to be fully resolved. Spore suspensions
(in saline containing the wetting agent Tween 80) obtained from 7-day-old
cultures are presently recommended. The species to be used may be a known
environmental strain and likely contaminant, such as Aspergillus niger, or a pathogen, such as Trichophyton mentagrophyes, other strains such as Penicillium variabile are also employed. Clearly
the final selection of organism will vary depending on the perceived use for
the disinfectant under test. In general, spore suspensions of at least 106 CFU/ml have been recommended. Viable counts
are typically performed on a suitable media (e.g. malt extract agar, sabouraud
dextrose agar) with incubation at 20°C for 48 hours or longer. EN 1275:1997
regulations for fungicidal activity require a minimum reduction in viability by
a factor of 104 within 60 minutes; test
fungi were Candida albicans and A. niger. Further procedures may be obtained by
reference to EN 1650:1998 (quantitative suspension test for evaluation of
fungicidal activity of chemical disinfectants and antiseptics used in food,
industrial, domestic and institutional areas) and AOAC Fungicidal activity of
disinfectants (955.17).
ii) Antiviral (viricidal) test
The evaluation of disinfectants for viricidal activity is a complicated
process requiring specialized training and facilities; viruses are obligate
intracellular parasites and are therefore incapable of independent growth and
replication in artificial culture media. They require some other system
employing living host cells. Suggested test viruses include rotavirus,
adenovirus, poliovirus, herpes simplex viruses, HIV, pox viruses and papova virus,
although extension of this list to include additional blood-borne viruses
such as hepatitis B and C, and significant animal pathogens (e.g. foot
and mouth disease virus) could be argued, given the potential impact on public
health or the economy of a nation.
Briefly, the virus is grown in an
appropriate cell line that is then mixed with water containing an organic load
and the disinfectant under test. After the appropriate time, residual viral
infectivity is determined using a tissue culture/plaque assay or other system
(e.g. animal host, molecular assay for some specific viral component). Such
procedures are costly and time-consuming, and must be appropriately controlled
to exclude factors such as disinfectant killing of the cell system or test
animal. A reduction of infectivity by a factor of 104 has been regarded as evidence of acceptable
viricidal activity (prEN 14476). For viruses that cannot be grown in the laboratory
(e.g. hepatitis B), naturally infected cells/tissues must be used. Further test
procedures are detailed in British Standard BS EN 13610 (quantitative
suspension test for the evaluation of viricidal activity against bacteriophages
of chemical disinfectants used in food and industrial areas). The use of
bacteriophage as model viruses in this procedure most likely reflects their
ease of growth and survivor enumeration via standard plaque assay on host
bacterial lawns grown on solid media.
i iii) Prion disinfection test
Prions are a unique class of acellular,
proteinaceous infectious agent, devoid of an agent-specific nucleic acid (DNA
or RNA). Infection is associated with the abnormal isoform of a host cellular
protein called prion protein (PrPc). Prions exhibit unusually high resistance
to conventional chemical and physical decontamination methods, presenting a
unique challenge in infection control. Although numerous published studies on
prion inactivation by disinfectants are available in the literature,
inconsistencies in methodology make direct comparison difficult. For example,
strain differences of prion (with respect to sensitivity to thermal and
chemical inactivation), prion concentration in tissue homogenate, exposure
conditions and determination of log reductions from incubation period assays
instead of end-point titrations. Furthermore, since most studies of prion
inactivation have been conducted with tissue homogenates, the protective effect
of the tissue components may offer some protective role and contribute to
resistance to disinfection approaches. Despite this, a consistent picture of
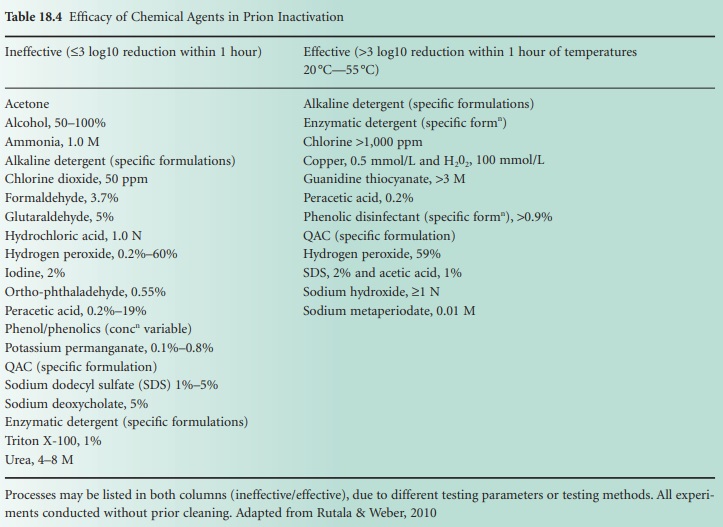
effective and ineffective agents has emerged and is summarized in Table 18.4.
Although most disinfectants are inadequate for the elimination of prion
infectivity, agents such as sodium hydroxide, a phenolic formulation, guanidine
thiocyanate and chlorine have all been shown to be effective.
Related Topics
