Forces Involved in Drug Receptors Interaction
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Receptors
Covalent bonds are the one which are produced by a strong energy.
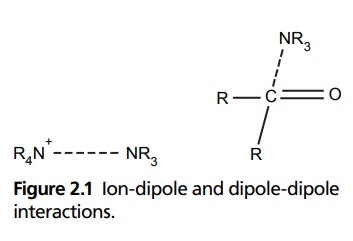
FORCES INVOLVED IN DRUG RECEPTORS INTERACTION Covalent bonds are the one which are produced by a strong energy. These are produced only when an irreversible antagonist inactivates the receptors. If a weak bond forms the drug receptor complex, the complex formed will be a reversible type. Example: Acetylcholinesterase is irreversibly inactivated by a number of phosphate esters (organophosphorus compounds includes pesticides). The nitrogen mustards are irreversible inhibitors of certain receptors. It is associated with electrostatic bonding. Molecules in which there is a partial charge separation between adjacent atoms or functional groups can interact either with each other or with ions. The carbon-X bonds in drugs and receptors (where X is an electronegative atom) will have asymmetric distribution of electrons. This produces electronic dipoles. The dipoles in a drug molecule can be attracted by ions (ion-dipole interaction) or by other dipole-dipole interaction (Fig. 2.1). Ion dipole interactions are more powerful and has high energy. It forms a weak (energy from 7 to 40 KJ/mol) and easily breaking bond. Since drugs contain hydroxyl, amino, carboxyl, and carbonyl groups it can form H bonds with the receptors. H-bonding is a type of dipole-dipole interaction formed between the proton of a group X-H (X-is an electronegative atom) and other electronegative atoms (Y) containing pairs of nonbonded electrons. X removes electron density from hydrogen. Therefore, it has a partial positive charge, which is strongly attached to nonbonded electrons of Y. Hydrogen bonding usually stabilizes the structures by intramolecular bond formation. Such bond formation occurs in protein alpha helix and in base pair of DNA. The opposite charged compound will interact with the opposite charged part of the receptor. The positively charged quaternary N-acetylcholine may be attracted to the negative charge of an ionized carboxyl group in the receptor. A dipole-dipole interaction is produced and a charge transfer complex is formed when any molecule with electron-donating property comes near the electron-accepting group. Donor molecules are electrons rich in heterocycles, for example, furan, thiophene, and aromatics with electron-donating substituents, or compounds with free nonbonding electron pair. Acceptor molecules are electron deficient systems such as purines and pyrimidines or aromatics with electron-withdrawing substituens. Examples of charge transfer complex in drug receptor interaction are antimalarials with their receptors and antibiotics intercalating the DNA. The energy is not more than 30 KJ/mol. In the presence of nonpolar molecules, the surrounding water molecules orient themselves, and therefore, are in a high-energy state, when two nonpolar receptor groups (one from the drug and another from the receptor) approach disorder–ordered H2O molecules in an attempt to associate with each other by increasing the enthalpy and decreasing the free energy to stabilize drug receptor-complex. Atoms in nonpolar molecular structure have a temporary nonsymmetrical distribution of electron density, which results in the generation of a temporary dipole that creates an intermolecule attraction called van der Waal’s force. However, this is a weak bond.Covalent Bonding
Dipole-Dipole and Ion-Dipole Interactions
Hydrogen Bonding
Electrostatic Bonding
Charge Transfer Complex
Hydrophobic Forces
van der Waal’s or London Dispersion Forces
Related Topics
