Packing and Labelling of Drug Formulations
| Home | | Forensic Pharmacy |Chapter: Forensic Pharmacy : The Drugs and Cosmetics Act (DCA) 1940 and Rules 1945
Different steps in the manufacturing of drugs are suitably monitored by way of provisions made under DCA and Rules.
Packing and Labelling of Drug
Formulations
Different steps in
the manufacturing of drugs are suitably monitored by way of provisions made
under DCA and Rules. The stages involved from procurement of raw material to
the sale of drug formulations at the retail counters are mandatorily controlled
in accordance with the provisions of the Act. In addition to mandatory
requirement, it is also the moral, ethical and social responsibility of the
manufacturer to ensure that the consumer receives good quality of his/her
products. Packing is a blend of art and science with regulatory flavour. It is
not sufficient to provide artistic packaging of the formulations but, it should
be sufficient enough to ensure the stability of product during transportation
and storage, assuring high quality of the product.
The
text of labelling on packing material varies with the type of product
formulated. There are specific requirements of labelling for drugs of Schedules
G, H and X; external
applications, patent and proprietary medicines, opthalmic ointments,
contraceptives, disinfectants and several other drug formulations.
Labelling
should be attractive and readable. It should be in printed form on the
outerside of the packing material, as well as, on the packing of drug
formulation. Even single unit offormulation (ampoule or tablet) should have
appropriate label on it. In case of single dose of tablet, it could be short
name of the product embossed on it.
The following
particulars should appear in the label of the drug formulation.
1.
Name (Patent or Proprietary and Generic name)
2.
Name and address of manufacturer
3.
Batch or lot number
4.
Date of manufacturing
5.
Expiry date, if any
6.
Information for storage, if any
7.
Precautionary information - i.e., care in handling the
product, use, etc.
8.
General information including - Physicians sample - not to
be sold, in case of, physicians.
In case of medicines
made up ready for treatment, name and address of licensee by whom it is
"Supplied should appear on the label.
In case of
preparations included in B.P, B.P.C, I.P the abbreviations should be mentioned.
In
a preparatron containing more than 3% alcohol, the percentage of alcohol should
be mentioned on the label.
Particulars of Label

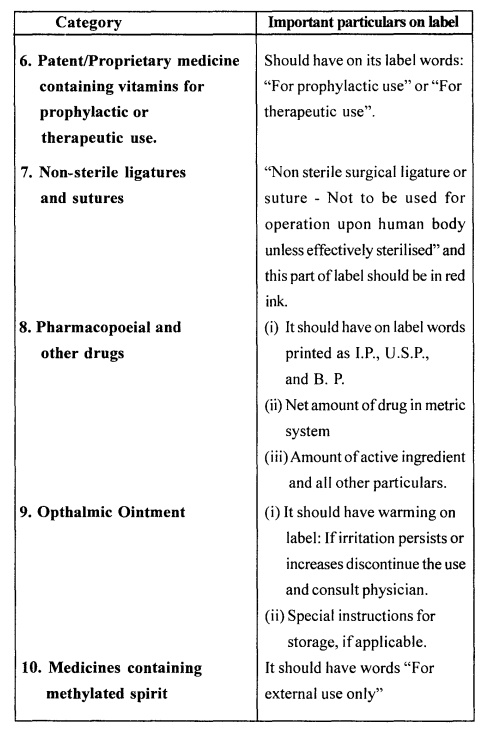
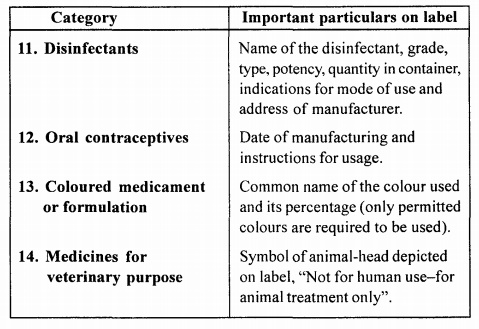
The label should be
printed or written in indelible ink and should clearly appear on label of inner
most container and every other covering of the container. The details of
labelling include-
1. Name ofthe drug
(Trade name/Generic name as applicable) should be printed clearly.
2. Net contents (weight, measure, volume or number of units
of activity as applicable). Net content should be in metric system.
3. Contents of active ingredients:
(a) For solid oral
dosage forms (tablet, capsule), content in each unit of formulation.
(b) Solid form for injectables, in terms of weight mg/gm of
powder. In case antibiotic, it is in terms of units of activity.
(c) Liquid orals: contents of ingredients in single dose of5
ml or multiple. If dose is less, the contents of ingredients in 1 ml of
preparation.
(d) Liquid parentral
preparation: contents of ingredients in I ml or per dose in case of single dose
preparation.
4.
Name and address of the manufacturer on small container. Name and place of
manufacture is sufficient on each ampoule
5. Manufacturing licence number abbreviated as Mfg. Lic. No.
6. Distinctive Batch No. should be written as Batch No. or
B.No. or Batch, Lot No.
7. Date of manufacturing.
8. Expiry particulars, ifany.
9. Precautions related to handling, use or distribution.
10. Information on storage.
11. Any other general information or specific information
pertaining to formulation. If the sample is for physician, the words
"Physicians sample - not for sale" should be printed.
For
Schedules F and F (I) and X drugs, only
Code No. as approved by the Licensing Authority is required to be printed.
Related Topics
