SAR of Cephalosporins
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antibiotics
The addition of amino group and a hydrogen to α and α1 position produces basic compound, which is protonated under acidic conditions of stomach.
SAR of Cephalosporins
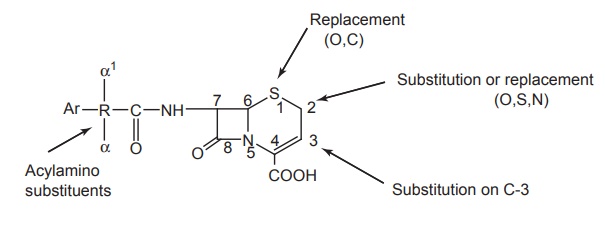
1. 7-Acylamino substitution
a. The
addition of amino group and a hydrogen to α and α1 position produces
basic compound, which is protonated under acidic conditions of stomach. The
ammonium ion improves the stability of β-lactum of cephalosporins and make
active orally. Activity against positive bacteria is increased and gram
negative is decreased by acylation of amino group.
b. When the
new acyl groups are derived from carboxylic acids, it shows good spectrum of
antibacterial action for gram-positive bacteria.
c. Substitutions
on the aromatic ring phenyl that increase lipophilicity provide higher
gram-positive activity and generally lower gram-negative activity.
d. The
phenyl ring in the side chain can be replaced with other heterocycles with
improved spectrum of activity and pharmacokinetic properties; these include
thiophene, tetrazole, furan, pyridine, and aminothiazoles.
e. The L-isomer
of an α-amino α1 -hydrogen derivative of cephalosphorins was 30–40
fold stable than D-isomer. Addition of methoxy oxime to α and α1increases
the stability to nearly 100-fold. The presence of catechol grouping can also
enhance activity, particularly, against Pseudomonas aeruginosa, and also retain
some gram-positive activity, which is unused for a catechol cephalosporin.
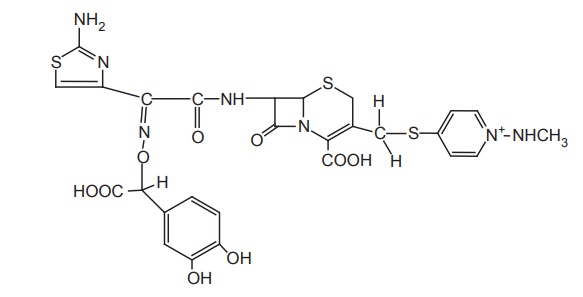
These
compounds penetrate into the cell by utilizing the bacterial ion β-dependent
ion transport system. There is a reduction of Gram negative activity when the
lipophilicity of this side chain is increased and effects of polar α-substituents
are enhanced (OH, NH2, SO3H, COOH).
2. Modification in the C-3 substitution: The pharmacokinetic and pharmacodynamics depends
on C-3 substituents. Modification at C-3 position has been made to reduce the
degradation (lactone of desacetyl cephalosporin) of cephalosporins.
a.
The benzoyl
ester displayers improved gram-positive activity, but lowered gram-negative
activity.
b.
Pyridine, imidaozle
replaced acetoxy group by azide ion yields derivative with relatively low
gramnegative activity.
c.
Displacement
with aromatic thiols of 3-acetoxy group results in an enhancement of activity
against gram-negative bacteria with improved pharmacokinetic properties.
d.
Orally
active compounds are produced by replacement of acetoxy group at C-3 position
with CH3 and Cl.
3. Other modifications
a.
Methoxy
group at C-7, shows higher resistance to hydrolysis by β-lactamase.
b.
Oxidation of
ring spectrum to sulphoxide or sulphone greatly diminishes or destroys the
antibacterial activity.
c.
Replacement
of sulphur with oxygen leads to oxacepam (latamoxet) with increased
antibacterial activity, because of its enhanced acylating power. Similarly,
replacement of sulphur with methylene group (loracavet) has greater chemical
stability and a longer half-life.
d.
The carboxyl
group position-4 has been converted into ester prodrugs to increase
bioavailability of cephalosporins, and these can be given orally as well.
e.
The
antibacterial activity depends on the olefinic linkage at C-3 and C-4 position
and their activity is lost due to the ionization of double bond to 2nd and 3rd
positions.
Related Topics
