Selective Serotonin Reuptake Inhibitors (SSRIs)
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antidepressants
Antidepressants - Selective Serotonin Reuptake Inhibitors (SSRIs) - Synthesis and Drug Profile- a. Fluoxetine (Fludac, Platin, Prodep) b. Fluvoxamine c. Sertraline - Structure, Properties, uses, Synthesis, Assay, Storage, Dosage forms, Dose | Synthesis and Drug Profile
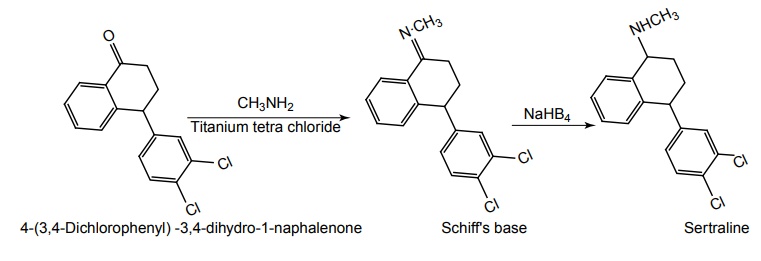
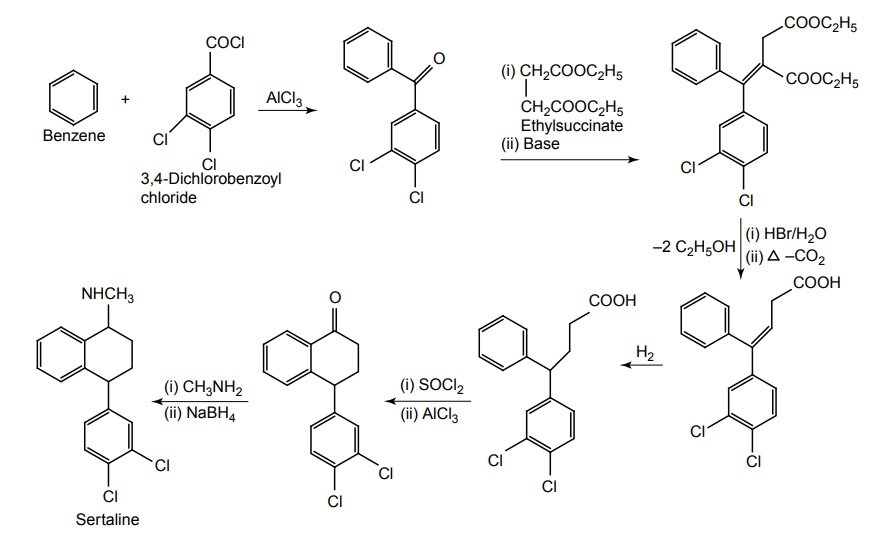
SYNTHESIS AND DRUG PROFILE Selective Serotonin Reuptake Inhibitors (SSRIs) Mode of Action: SSRIs have analogues action to TCAs at serotonin containing neurons. Selective serotonin reuptake inhibitors, inhibits reuptake of serotonin. After serotonin is released from neuron, it is removed from the extracellular space by transporters or reuptake sites, located on the cell membrane, SSRIs blocks serotonin reuptake to remain active in the synaptic binding site for longer time. Following the release of serotonin in nerve terminals, it interacts with postsynaptic 5-HT1-7 receptors and exerts their effects through a variety of phospholipase and cyclase-mediated mechanisms. Inhibitory auto receptors include 5-HT1A and perhaps 5-HT7 subtypes at serotonin cell bodies as well as 5-HT1D receptors in the nerve terminals, and dendrites become desensitized following prolonged treatment with SSRIs and enhances the excitatory function. Structurally, the SSRIs differ from the tricyclic in that the centre ring of tricyclic has been taken apart. The net effect is that the aryl amine similar to the grouping is present as in the tricyclics and the compound can compete for the substrate binding site of the serotonin transporter protein. As in the tricyclics the extra acyl group can add extra affinity and give favourable competition with the substrate serotonin. (±)-3-(p-Trifluoro methyl phenoxy)-N-methyl-3-phenyl-propylamine. Synthesis Route I. From: Beta (methyl amino) propiophenone Route II. From: Acetophenone Properties and uses: It is a white or almost white crystalline powder, soluble in methanol, sparingly soluble in water, and in methylene chloride. It is a selective serotonin reuptake inhibitor and antidepressant. It is used for the treatment of endogeneous depression. It may be useful in treating obsessive-compulsive disorder, obesity, and alcoholism. It is a selective inhibitor of serotonin uptake in the CNS and has little effect on the control of NE or dopamine function. Assay: Assayed by liquid chromatography method. Dose: The usual dose is 20–80 mg/day. Dosage forms: Fluoxetine capsules B.P., Fluoxetine oral solution B.P. Synthesis Properties and uses: It is a white to almost white crystalline powder, sparingly soluble in water, freely soluble in ethanol, and in methanol. It is a selective serotonin reuptake inhibitor, antidepressant, and effective in treating depressive disorders. It may be relatively safe to use in depressed individuals with cardiaovascular diseases. The most common adverse effects are nausea, headache, dry mouth, and insomnia. Assay: It is assayed by titrating with 0.1 M perchloric acid (nonaqueous titration) and the end point is determined potentiometrically. Storage: It should be stored in well-closed airtight containers. Dosage forms: Fluvoxamine tablets B.P. 4-[3, 4-Dichloro phenyl] 1, 2, 3, 4-tetra hydro-N-methyl naphthalamine Synthesis Route I. From: 4-(3,4-Dichlorophenyl)-3,4-dihydro-1-naphthalenone Route II. From: Benzene and 3, 4-dichlorobenzoyl chloride Properties and uses: Sertraline works by blocking presynaptic reuptake of serotonin and is considerably more potent in this effect than any antidepressant of this class. It is twice as potent in its inhibition of serotonin reuptake activity than in its inhibition of NE or dopamine uptake inhibiton. Used as selective serotonin reuptake inhibitor with low sedative and antimuscarnic activity, also used for panic disorder and premenstrual dysphonic disorder.a. Fluoxetine (Fludac, Platin, Prodep)
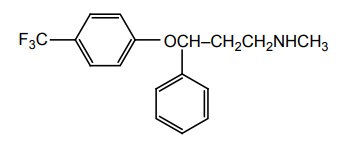
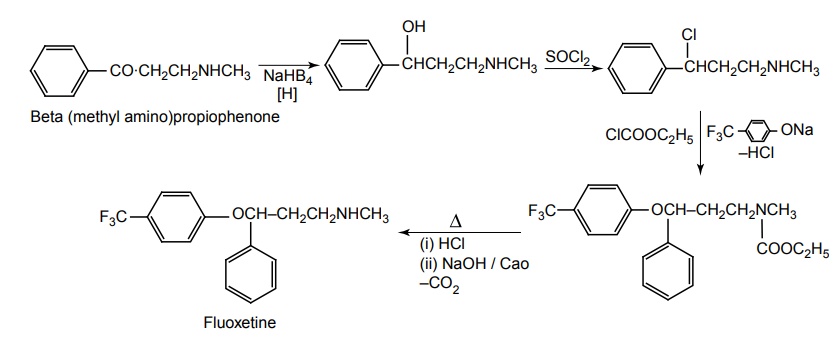

b. Fluvoxamine

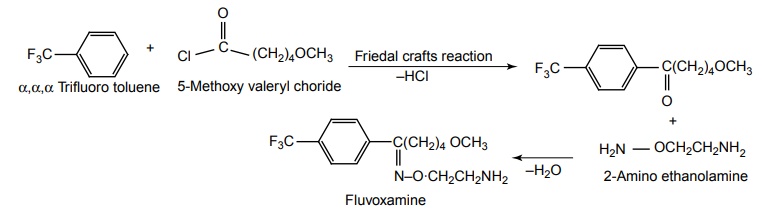
c. Sertraline
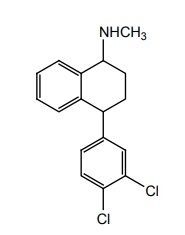
Related Topics
