Sources and Control of Contamination
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Microbial Spoilage, Infection Risk And Contamination Control
Regardless of whether manufacture takes place in industry or on a smaller scale in the hospital pharmacy, the microbiological quality of the finished product will be determined by the formulation components used, the environment in which they are manufactured and the manufacturing process itself.
SOURCES AND CONTROL OF CONTAMINATION
A) In Manufacture
Regardless of whether manufacture takes place in industry or on a
smaller scale in the hospital pharmacy, the microbiological quality of the
finished product will be determined by the formulation components used, the
environment in which they are manufactured and the manufacturing process itself.
Quality must be built into the product at all stages of the process and not
simply inspected at the end of manufacture:
• Raw materials, particularly water and those of natural origin, must be
of a high microbiological standard.
• All processing equipment should be subject to planned preventive
maintenance and should be properly cleaned after use to prevent
cross-contamination between batches.
• Cleaning equipment should be appropriate for the task in hand and
should be thoroughly cleaned and properly maintained.
• Manufacture should take place in suitable premises, supplied with
filtered air, for which the environmental requirements vary according to the
type of product being made.
• Staff involved in manufacture should not only have good health but
also a sound knowledge of the importance of personal and production hygiene.
• The end-product requires suitable packaging which will protect it from
contamination during its shelf-life and is itself free from contamination.
a)
Hospital Manufacture
Manufacture in hospital premises raises certain additional problems with
regard to contamination control.
i)
Water
Mains water in hospitals is frequently stored in large roof tanks, some
of which may be relatively inaccessible and poorly maintained. Water for
pharmaceutical manufacture requires some further treatment, usually by
distillation, reverse osmosis or deionization or a combination of these,
depending on the intended use of water. Such processes need careful monitoring,
as does the microbiological quality of the water after treatment. Storage of
water requires particular care, as some Gram-negative opportunist pathogens can
survive on traces of organic matter present in treated water and will readily
multiply to high numbers at room temperature. Water should therefore be stored
at a temperature in excess of 80 °C and circulated in the distribution system
at a flow rate of 1–2 m/s to prevent the build-up of bacterial biofilms in the
piping.
ii)
Environment
The microbial flora of the hospital pharmacy environment is a reflection
of the general hospital environment and the activities undertaken there.
Free-living opportunist pathogens, such as Ps. aeruginosa, can
normally be found in wet sites, such as drains, sinks and taps. Cleaning
equipment, such as mops, buckets, cloths and scrubbing machines, may be
responsible for distributing these organisms around the pharmacy; if stored wet
they provide a convenient niche for microbial growth, resulting in heavy
contamination of equipment. Contamination levels in the production environment
may, however, be minimized by observing good manufacturing practices (GMP), by
installing heating traps in sink U-bends, thus destroying one of the main reservoirs
of contaminants, and by proper maintenance and storage of equipment, including
cleaning equipment. Additionally, cleaning of production units by contractors
should be carried out to a pharmaceutical specification.
iii)
Packaging
Sacking, cardboard, card liners, corks and paper are unsuitable for
packaging pharmaceuticals, as they are heavily contaminated, for example with
bacterial or fungal spores. These have now been replaced by nonbiodegradable
plastic materials. In the past, packaging in hospitals was frequently reused
for economic reasons. Large numbers of containers may be returned to the
pharmacy, bringing with them microbial contaminants introduced during use in
the wards. Particular problems have been encountered with disinfectant solutions
where residues of old stock have been ‘topped up’ with fresh supplies,
resulting in the issue of contaminated solutions to wards. Reusable containers
must therefore be thoroughly washed and dried, and never refilled directly.
Another common practice in hospitals is the repackaging of products
purchased in bulk into smaller containers. Increased handling of the product
inevitably increases the risk of contamination, as shown by one survey when
hospital-repacked items were found to be contaminated twice as often as those
in the original pack (Public Health Laboratory Service Report, 1971).
B) In Use
Pharmaceutical manufacturers may justly argue that their responsibility
ends with the supply of a well-preserved product of high microbiological
standard in a suitable pack and that the subsequent use, or indeed abuse, of
the product is of little concern to them. Although much less is known about how
products become contaminated during use, their continued use in a contaminated
state is clearly undesirable, particularly in hospitals where it could result
in the spread of cross-infection. All multidose products are vulnerable to
contamination during use. Regardless of whether products are used in hospital
or in the community environment, the sources of contamination are the same, but
opportunities for observing it are greater in the former. Although the risk of
contamination during product use has been much reduced in recent years,
primarily through improvements in packaging and changes in nursing practices,
it is nevertheless salutary to reflect upon past reported case histories.
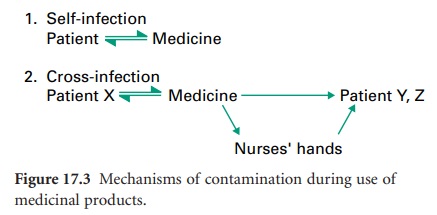
b) Human Sources
During normal usage, patients may contaminate their medicine with their
own microbial flora; subsequent use of such products may or may not result in
self-infection (Figure 17.3).
Topical products are considered to be most
at risk, as the
product will probably
be applied by hand, thus
introducing contaminants from
the resident skin
flora of staphylococci, Micrococcus spp.
and diphtheroids but also
perhaps transient
contaminants, such as Pseudomonas or coliforms, which would normally
be removed with effective hand-washing. Opportunities for contamination may be reduced
by using disposable applicators for topical products or by giving
oral products by disposable
spoon.
In hospitals, multidose
products, once contaminated, may serve as a vehicle
of cross-contamination or cross-infection between patients. Zinc-based products
packed in large stockpots and used in the treatment
and prevention of bedsores in long-stay and geriatric patients were reportedly contaminated during use with Ps. aeruginosa and Staphylococcus aureus. If unpreserved, these products
permit multiplication of contaminants, especially if water is present either
as part of the formulation, for example in oil/water (o/w) emulsions, or as a film in w/o emulsions
which have undergone local
cracking, or as a condensed film from atmospheric water. Appreciable numbers of contaminants may then be transferred to other patients when the product is reused. Clearly
the economics and convenience of using stockpots need to be balanced against
the risk of spreading cross-infection between patients and the inevitable increase in length of the patients’ stay in hospital. The use of stockpots
in hospitals
has noticeably declined
over the past two
decades or so.
A further
potential source of contamination in hospitals is the nursing staff responsible for medicament
administration. During
the course of their work, nurses’ hands become contaminated with opportunist pathogens which are not
part of the normal skin
flora but which
are easily removed
by thorough hand-washing and drying. In busy
wards, hand-washing between
attending to patients may be overlooked and
contaminants may subsequently be transferred to medicaments during administration.
Hand lotions and creams used to prevent
chapping of nurses’ hands may similarly become
contaminated, especially when packaged
in multidose containers and left at the side of the hand-basin, frequently without lids.
Hand lotions and creams should be well preserved
and, ideally, packaged in disposable dispensers. Other effective control methods include the supply of products in individual patient’s
packs and the use of non-touch techniques for medicament administration. The importance of thorough hand-washing in the control
of hospital cross-infection cannot be overemphasized. In recent
years hospitals have successfully raised
the level of awareness
on this
topic among staff
and the general
public through widespread publicity and the provision of
easily accessible hand disinfection stations
on the wards.
c)
Environmental Sources
Small numbers
of airborne contaminants may settle in products left open to the atmosphere. Some of these
will die during storage, with the rest
probably remaining at a
static level of about 102–103 colony
forming units (CFU) per gram or per millilitre. Larger
numbers of waterborne contaminants
may be accidentally introduced into topical
products by wet hands
or by a ‘splash-back mechanism’ if left at the side of a basin.
Such contaminants generally have simple nutritional
requirements and, following multiplication, levels of contamination may often exceed 106 CFU/g.
In the past this problem
has been encountered particularly
when the product
was stored in warm hospital wards or in hot steamy bathroom
cupboards at home. Products used in hospitals
as soap substitutes for
bathing patients are particularly at risk and soon not only
become contaminated with opportunist pathogens such as Pseudomonas spp.,
but also provide
conditions conducive to their
multiplication. The problem
is compounded by stocks kept
in multidose pots
for use by several patients in the same ward over an extended
period of time.
The indigenous microbial population is quite different in the home
and in hospitals. Pathogenic organisms are found much more
frequently in the latter and
consequently are isolated more often from medicines used in
hospital. Usually, there are fewer opportunities for contamination in the home, as patients are generally issued with individual supplies in small quantities.
d) Equipment Sources
Patients and nursing staff may use a range of applicators (pads, sponges, brushes
and spatulas) during
medicament administration, particularly for topical products. If reused, these easily become contaminated and may be responsible for perpetuating contamination
between fresh
stocks of product, as has indeed been
shown in studies of
cosmetic products. Disposable applicators or swabs should therefore
always be used.
In hospitals today a wide
variety of complex
equipment is used in the course of patient treatment. Humidifiers, incubators, ventilators,
resuscitators and other apparatus require
proper maintenance and decontamination after
use. Chemical disinfectants used for this purpose have in the past,
through misuse, become
contaminated with opportunist pathogens, such as Ps. aeruginosa, and ironically have contributed to, rather than
reduced, the spread of cross-infection in hospital patients. Disinfectants should only be used for their
intended purpose and directions for use must be followed
at all times.
Related Topics
