Sterol or Cardiac Glycosides
| Home | | Pharmacognosy |Chapter: Pharmacognosy and Phytochemistry : Drugs Containing Glycosides
The cardiac glycosides are an important class of naturally occurring drugs whose actions include both beneficial and toxic effects on the heart. Plants containing cardiac steroids have been used as poisons and heart drugs at least since 1500 B.C.
STEROL OR CARDIAC GLYCOSIDES
The cardiac glycosides are an important class of naturally
occurring drugs whose actions include both beneficial and toxic effects on the
heart. Plants containing cardiac steroids have been used as poisons and heart
drugs at least since 1500 B.C. Throughout history these plants or their
extracts have been variously used as arrow poisons, emetics, diuretics and
heart tonics. Cardiac steroids are widely used in the modern treatment of
congestive heart failure and for treatment of atrial fibrillation and flutter.
Yet their toxicity remains a serious problem. These drugs all act by affecting
the availability of intracellular Ca+2 for myocardial contraction or
increasing the sensitivity of myocardial contractile proteins.
Cardiac glycosides are composed of two structural fea-tures:
the sugar (glycone) and the nonsugar (aglycone– steroid) moieties.
The steroid nucleus has a unique set of fused ring system
that makes the aglycone moiety structurally distinct from the other more common
steroid ring systems. The steroid nucleus has hydroxyls at 3- and 14-positions
of which the sugar attachment uses the 3-OH group. 14-OH is normally
unsubstituted. Many genins have OH groups at 12- and 16-positions. These
additional hydroxyl groups influence the partitioning of the cardiac glycosides
into the aqueous media and greatly affect the duration of action. The lactone
moiety at C-17 position is an important structural feature. The size and degree
of unsaturation varies with the source of the glycoside. Normally plant sources
provide a five-membered unsaturated lactone while animal sources give a
six-membered unsaturated lactone.
One to four sugars are found to be present in most cardiac
glycosides attached to the 3β-OH group. The sugars most commonly
used include L-rhamnose, D-glucose, D-digitoxose, D-digitalose, D-digginose,
D-sarmentose, L-vallarose and D-fructose. These sugars predominantly exist in
the cardiac glycosides in the β-conformation. The presence of acetyl
group on the sugar affects the lipophilic character and the kinetics of the
entire glycoside.
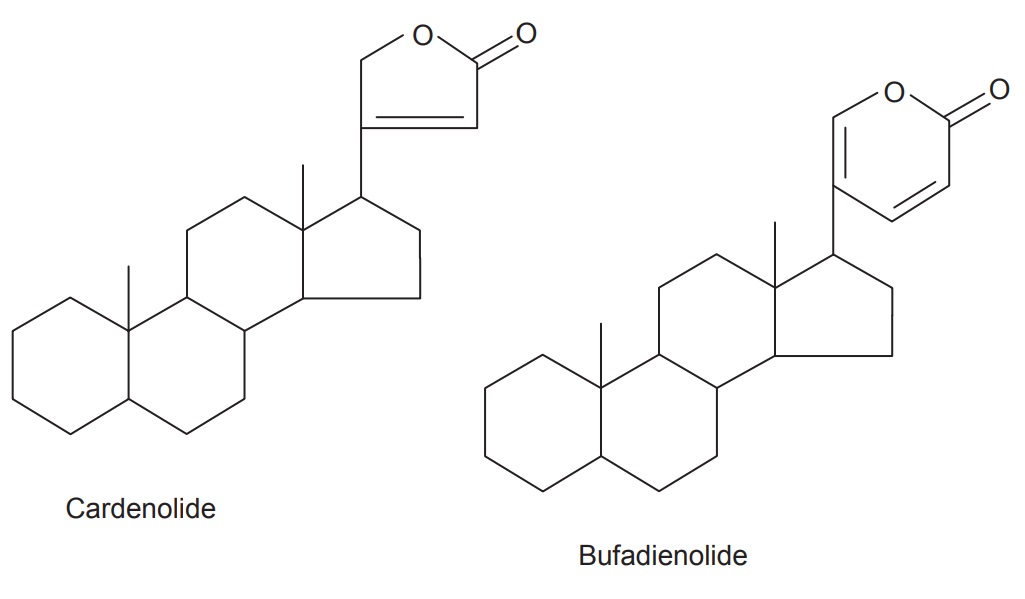
Two classes have been observed in nature—the cardenolides
and the bufadienolides.
The cardenolides have an unsaturated butyrolactone ring
while the bufadienolides have a pyrone ring. The lactone of cardenolides has a
single double bond and is attached at the C-17 position of steroidal nucleus.
They are five-membered lactone ring and form a C23 steroids
(Leguminosae, Cruciferae, Euphorbiaceae, etc.), while the lactone of
bufadienolids have two double bond which is attached at the 17 α-position of the steroidal nucleus. They are six-memberd
lactone ring and form C24 steroids (Liliaceae, Ranunculaceae).