Sulfonamides
| Home | | Pharmacology |Chapter: Essential pharmacology : Sulfonamides, Cotrimoxazole And Quinolones
Sulfonamides were the first antimicrobial agents (AMAs) effective against pyogenic bacterial infections. Sulfonamido-chrysoidine (Prontosil Red) was one of the dyes included by Domagk to treat experimental streptococcal infection in mice and found it to be highly effective.
SULFONAMIDES
Sulfonamides were the
first antimicrobial agents (AMAs) effective against pyogenic bacterial
infections. Sulfonamido-chrysoidine (Prontosil Red) was one of the dyes
included by Domagk to treat experimental streptococcal infection in mice and
found it to be highly effective. He subsequently cured his daughter of
streptococcal septicaemia (which was 100% fatal at that time) by prontosil. By
1937, it became clear that prontosil was broken down in the body to release
sulfanilamide which was the active antibacterial agent. A large number of
sulfonamides were produced and used extensively in the subsequent years, but
because of rapid emergence of bacterial resistance and the availability of many
safer and more effective antibiotics, their current utility is limited, except
in combination with trimethoprim (as cotrimoxazole) or pyrimethamine (for
malaria).
Chemistry
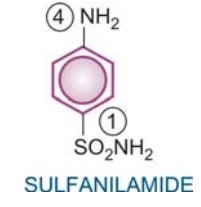
All sulfonamides may
be considered to be derivatives of sulfanilamide
(p-aminobenzene sulfonamide). Individual members differ in the nature of N1
(Sulfonamido N) substitution, which governs solubility, potency and
pharmacokinetic property. A free amino group in the para position (N4) is
required for antibacterial activity.
Sulfonamides that are
still of clinical interest are:
1.
Short acting (4–8 hr): Sulfadiazine
2.
Intermediate acting (8–12 hr): Sulfamethoxazole
3.
Long acting (~7 days): Sulfadoxine, Sulfamethopyrazine
4. Special purpose sulfonamides: Sulfacetamide sod., Mafenide, Silver
sulfadiazine, Sulfasalazine
ANTIBACTERIAL SPECTRUM
Sulfonamides are
primarily bacteriostatic against many gram-positive and gram-negative bacteria.
However, bactericidal concentrations may be attained in urine. Sensitivity patterns
among microorganisms have changed from time-to-time and place-to-place. Those
still sensitive are:
many Strepto. pyogenes, Haemophilus influenzae,
H. ducreyi, Calymmatobacterium
granulomatis, Vibrio cholerae. Only a few Staph. aureus, gonococci, meningococci, pneumococci, Escherichia coli, and Shigella respond, but majority are resistant.
Anaerobic bacteria are not susceptible.
Chlamydiae: trachoma,
lymphogranuloma venereum, inclusion conjunctivitis, are sensitive, as are Actinomyces, Nocardia and Toxoplasma.
Mechanism Of Action
Many bacteria synthesize their own folic acid (FA) of which p-aminobenzoic
acid (PABA) is a constituent, and is taken up from the medium. Woods and Fildes
(1940) proposed the hypothesis regarding sulfonamide action. Sulfonamides,
being structural analogues of PABA, inhibit bacterial folate synthase → FA is not formed and
a number of essential metabolic reactions suffer. Sulfonamides competitively
inhibit the union of PABA with pteridine residue to form dihydropteroic acid
which conjugates with glutamic acid to produce dihydrofolic acid. Also, being chemically
similar to PABA, the sulfonamide may itself get incorporated to form an altered
folate which is metabolically injurious.
Human cells also require FA, but they utilize preformed FA
supplied in diet and are unaffected by sulfonamides. Evidences in favour of
this mechanism of action of sulfonamides are:
a)
PABA, in small quantities, antagonizes the
antibacterial action of sulfonamides.
b) Only those microbes which synthesize their own
FA, and cannot take it from the medium are susceptible to sulfonamides.
Pus and tissue extracts contain purines and thymidine which
decrease bacterial requirement for FA and antagonize sulfonamide action. Pus is
also rich in PABA.
Resistance To Sulfonamides
Most bacteria are capable of developing resistance to sulfonamides.
Prominent among these are gonococci, pneumococci, Staph. aureus, meningococci, E.
coli, Shigella and some Strep.
pyogenes, Strep. viridans and anaerobes. The resistant mutants either:
a)
produce increased amounts of PABA, or
b)
their folate synthase enzyme has low affinity
for sulfonamides, or
c)
adopt an alternative pathway in folate
metabolism.
Resistance developed in vivo is quite persistent. Sensitivity
patterns have changed depending on the extent of use. When an organism is
resistant to one sulfonamide, it is resistant to them all. No cross resistance
between sulfonamides and other AMAs has been noted. Development of resistance
has markedly limited the clinical usefulness of this class of compounds.
Pharmacokinetics
Sulfonamides are
rapidly and nearly completely absorbed from g.i.t. Extent of plasma protein
binding differs considerably (10–95%) among different members. The highly
protein bound members are longer acting. Sulfonamides are widely distributed in
the body—enter serous cavities easily. The free form of sulfadiazine attains
the same concentration in CSF as in plasma. They cross placenta freely.
The primary pathway of
metabolism of sulfonamides is acetylation at N4 by non-microsomal acetyl transferase,
primarily in liver. There are slow and fast acetylators, but the difference is
mostly insufficient to be clinically significant. The extent of metabolism
differs for different members. The acetylated derivative is inactive, but can
contribute to the adverse effects. It is generally less soluble in acidic urine
than the parent drug—may precipitate and cause crystalluria. The acetylated
form accumulates in blood in patients with renal failure along with the parent
drug—toxicity increases.
Sulfonamides are
excreted mainly by the kidney through glomerular filtration. Both renal tubular
secretion and reabsorption also occur. The more lipidsoluble members are highly
reabsorbed in the tubule, therefore are longer acting.
Sulfadiazine
It is the prototype of
the general purpose sulfonamides that is
rapidly absorbed orally and rapidly excreted in urine. It is 50% plasma protein
bound and 20–40% acetylated. The acetylated derivative is less soluble in
urine, crystalluria is likely. It has good penetrability in brain and CSF—was
the preferred compound for meningitis.
Dose: 0.5 g QID to 2 g TDS; SULFADIAZINE 0.5 g
tab.
Sulfamethoxazole
It has slower oral
absorption and urinary
excretion—intermediate duration of action, t½ in adults averages 10 hours. It is
the preferred compound for combining with trimethoprim because the t½ of both
is similar. However, a high fraction is acetylated, which is relatively
insoluble—crystalluria can occur.
Dose: 1 g BD for 2 days,
then 0.5 g BD.
GANTANOL 0.5 g tab.
Sulfadoxine, Sulfamethopyrazine
These are ultralong acting compounds, action lasting > 1 week
because of high plasma protein binding and slow renal excretion (t½ 5–9 days).
They attain low plasma concentration (of free form) and are not suitable for
treatment of acute pyogenic infections. They are used in combination with
pyrimethamine in the treatment of malaria, (especially chloroquine resistant P. falciparum ; See Ch. No. 59), Pneumocystis
jiroveci pneumonia in AIDS patients and in toxoplasmosis. Because they have
caused serious cutaneous reactions, largescale use of the combination for
prophylaxis of malaria is not recommended.
Sulfacetamide sod.
It is a highly soluble
compound yielding neutral
solution which is only mildly irritating to the eye in concentrations up to
30%. It is used topically for ocular infections due to susceptible bacteria and
chlamydia, including ophthalmia neonatorum caused by Ch. No. oculogenitalis. It attains high concentrations in anterior segment and aqueous humour after
topical instillation. The incidence of sensitivity reactions with ocular use of
sulfacetamide sod. has been low; but it must be promptly stopped when they
occur.
LOCULA, ALBUCID 10%,
20%, 30% eye drops, 6% eye oint.
Mafenide
It is not a typical
sulfonamide, because a — CH2— bridge
separates the benzene ring and the amino group. It is used only
topically—inhibits a variety of gram-positive and gram-negative bacteria. In
contrast to typical sulfonamides, it is active in the presence of pus and
against Pseudomonas, clostridia which
are not inhibited by typical sulfonamides.
It has been mainly employed for burn dressing to prevent infection, but not to
treat already infected cases.
The biggest limitation
is that mafenide produces burning sensation and severe pain when applied to raw
surface. It is rapidly absorbed from the raw surface, metabolized and excreted
in urine. Mafenide and its metabolite are carbonic anhydrase (CAse) inhibitors—
alkalinize urine, can cause acidosis and hyperventilation: must not be applied
over large areas. Allergic reactions, particularly rashes also occur.
SULFAMYLON 1% cream
for surface application.
Silver Sulfadiazine
Used topically as 1%
cream, it is active against a large
number of bacteria and fungi, even those resistant to other sulfonamides, e.g. Pseudomonas. It slowly releases silver
ions which appear to be largely responsible for the antimicrobial action. It is
considered to be one of the most effective drugs for preventing infection of
burnt surfaces and chronic ulcers and is well tolerated. However, it is not
good for treating established infection. SILVIRIN 1% cream,
ARGENEX 1% cream with chlorhexidine 0.2%.
Local side effects
are—burning sensation on application and itch.
Released sulfadiazine
may be absorbed systemically and produce its own adverse effects.
Sulfasalazine used in ulcerative
colitis and rheumatoid
arthritis.
Adverse Effects
Adverse effects to
sulfonamides are relatively common. These are:
· Nausea, vomiting and epigastric pain.
· Crystalluria is dose related, but infrequent
now. Precipitation in urine can be minimized by taking plenty of fluids and by
alkalinizing the urine in which sulfonamides and their acetylated derivatives
are more soluble.
· Hypersensitivity reactions occur in 2–5% patients.
These are mostly in the form of rashes, urticaria and drug fever. Photosensitization
is reported. Stevens-Johnson Syndrome and exfoliative dermatitis are more
common with long-acting agents.
· Hepatitis, unrelated to dose, occurs in 0.1%
patients.
· Topical use of sulfonamides is not recommended
because of risk of contact sensitization. However, ocular use is permitted.
· Sulfonamides cause haemolysis in a dose-dependent
manner in individuals with G6PD deficiency. Neutropenia and other blood dyscrasias
are rare.
· Kernicterus may be precipitated in the newborn,
especially premature, by displacement of bilirubin from plasma protein binding
sites and more permeable blood-brain barrier.
Interactions
Sulfonamides inhibit
the metabolism (possibly displace from protein binding also) of phenytoin,
tolbutamide and warfarin— enhance their action.
They displace
methotrexate from binding and decrease its renal excretion—toxicity can occur.
Fixed dose
combinations of sulfonamides with penicillin are banned in India.
Uses
Systemic use of sulfonamides alone (not combined with trimethoprim
or pyrimethamine) is rare now. Though they can be employed for suppressive
therapy of chronic urinary tract infection, for streptococcal pharyngitis and
gum infection; such uses are outmoded.
Combined with
trimethoprim (as cotrimoxazole) sulfamethoxazole is used for many bacterial
infections, P. jiroveci and
nocardiasis (see below). Along with
pyrimethamine, certain sulfonamides are used for malaria (see Ch. No. 59) and toxoplasmosis.
Ocular sulfacetamide sod. (10–30%) is a cheap alternative in trachoma/inclusion
conjunctivitis, though additional systemic azithromycin or tetracycline therapy
is required for eradication of the disease. Topical silver sulfadiazine or
mafenide are used for preventing infection on burn surfaces.
Related Topics
