Supplementary prescribing
| Home | | Hospital pharmacy |Chapter: Hospital pharmacy : Pharmacist prescribing
Supplementary prescribing involves a partnership between a medical practi-tioner (independent prescriber) who establishes the diagnosis and initiates treatment.
Supplementary prescribing
Supplementary
prescribing involves a partnership between a medical practi-tioner (independent
prescriber) who establishes the diagnosis and initiates treatment, a
supplementary prescriber who monitors the patient and pre-scribes further
supplies of medication and the patient who agrees to the supplementary
prescribing arrangement.
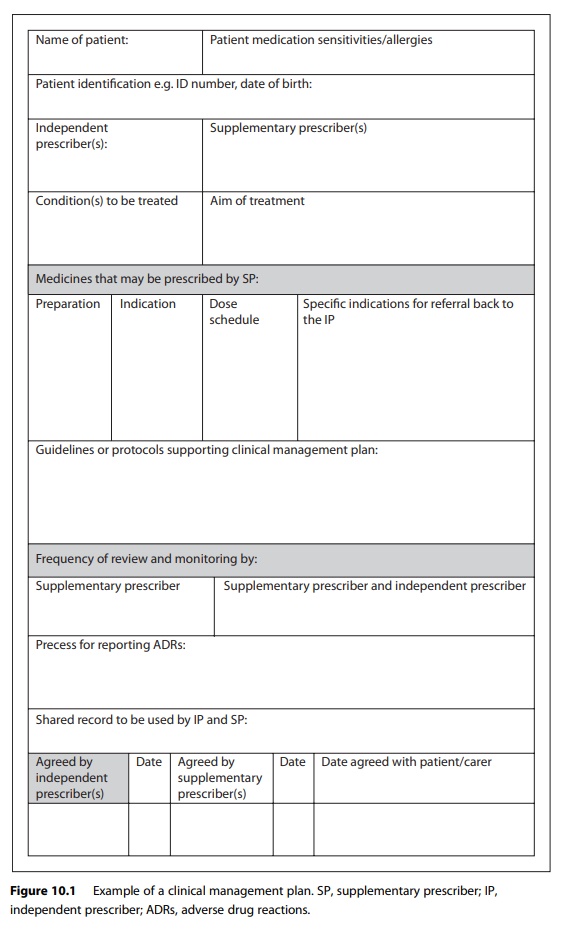
A clinical
management plan is drawn up for each patient which includes demographic data,
the condition to be treated, the treatment with medicines and information
detailing when the patient should be referred back to the independent
prescriber. Figure 10.1 provides an example template for a clin-ical management
plan; other examples are available online. Prescribing information included in
clinical management plans can refer to an agreed limited amount of information
and dosage adjustments that the supplemen-tary prescriber can make or, more
usually, to a locally or nationally agreed clinical guideline or pathway, for
example, the British Thoracic Society’s asthma guidelines.
The supplementary
prescribing model is the one initially recommended for pharmacists by the Crown
report and subsequently put into statute in 2003. The profession found this
model to be somewhat unwieldy and pursued independent prescribing; this was made
possible in May 2006.
Supplementary
prescribing is championed as the efficient model for the management of
long-term therapies within primary and secondary care. It has been used in
hospital practice to establish pharmacists within out-patient clinics and
provided a sound basis on which to build prescribing practice for pharmacists.
A supplementary prescriber, using a clinical management plan, may prescribe a
wide range of items, summarised in Text box 10.2.
Box 10.2 Medicines available to pharmacist
supplementary prescribers
Pharmacist
supplementary prescribing
Any
general sales list, pharmacy or prescription-only medicine prescribable at
National Health Service expense. This includes the prescribing of:
•
antimicrobials
•
‘black triangle’ drugs and those products suggested by the British National
Formulary to be ‘less suitable’ for prescribing
•
controlled drugs (except those listed in schedule 1 of The Misuse of Drugs
Regulations 2001 that are not intended for medicinal use)
•
products used outside their UK-licensed indications (known as ‘off-label’ use).
Such use must have the joint agreement of both prescribers and the status of
the drug should be recorded in the clinical management plan
•
unlicensed medicines
Related Topics
