Support for decision-making on new medicines
| Home | | Hospital pharmacy |Chapter: Hospital pharmacy : Medicines information
New medicines can offer benefits to patients and to the NHS. However, whilst new medicines may result in potential savings in some parts of the care pathway, they are often more expensive than established drug therapies.
Support for decision-making on new medicines
New medicines can
offer benefits to patients and to the NHS. However, whilst new medicines may
result in potential savings in some parts of the care pathway, they are often
more expensive than established drug therapies. Not all new medicines have
advantages over existing therapy and generally, where the benefits exist, they
are incremental rather than significant. As a result, the NHS has to manage the
introduction of new medicines carefully, taking into account their clinical and
cost-effectiveness. A number of bodies have been established in the UK to help
the NHS manage this process by conducting technology appraisals. In England
(and Wales), NICE assesses an increasing number of newly licensed medicines.
Where NICE appraises a medicine positively, the NHS is required to adopt the
medicine and make funding available for the indications for which it has been
approved. In Scotland the equivalent body is SMC; in Wales, the AWMSG provides
guid-ance in addition to that provided by NICE. The SMC and the AWMSG make
recommendations for the use of new medicines within their populations but these
do not have the statutory enforcement of NICE Technology Appraisals.
Where there is no
national guidance, the decision on the introduction and funding of a new
medicine has to be made locally by a trust medicines management committee (drug
and therapeutics committee or area prescribing committee). Lord Darzi’s Next
Stage Review.] and The NHS Constitution acknowledged that patients should have
access to the most clinically and cost-effective medicines and have the right
to ‘expect rational local decisions on funding of new drugs and treatments’.
The robustness of the evidence base on which these committees make their
decisions is therefore of great importance. Chapter 11 deals with these issues
in greater depth.
In many hospitals,
the MI staff support their medicines management committee by providing the
evidence on which the committee will make a decision. In some cases, where no
other evaluation is available, the trust MI pharmacist may critically appraise
the evidence for the committee. However, wherever possible, MI pharmacists will
use evaluations produced by credible other bodies, including those produced by
the UKMi network, as part of the UKMi ‘new product portfolio’ of resources.
Information on newly launched medicines
The UKMi evaluations
of new products are published under the title of New Medicines Profiles
(http://www.ukmi.nhs.uk/Med_info/profile.asp). Medicines considered for
evaluation include the first or second new medicine in a therapeutic class, and
medicines with major new indications or major new formulations. The aim of the
profiles is to assess the available evidence on efficacy and safety of a drug
and to review its place in therapy and any risk management issues. The
evaluations are written to strict criteria to ensure quality and accuracy, and
comments are invited from relevant clinicians and from the pharmaceutical
manufacturer before publication. In addition to supporting the needs of
decision-making committees, they are also intended to support the information
needs of clinicians and prescribers.
In order to prevent
duplication of work and to extend the range of medi-cines for which evaluations
are available, UKMi takes account of the work produced by other organisations,
including the National Prescribing Centre (NPC) and the London New Drugs Group
(LNDG) when deciding if a New Medicines Profile is to be written.
Planning for new medicines
Advanced notice and
planning for the introduction of new medicines are essential if NHS
organisations are to allocate resource appropriately. The number of high-cost
treatments, the NHS Constitution and increasingly vocal interest groups make
the task of allocating resources one of the most politically sensitive and
complex issues facing the NHS.
Prescribing Outlook,
New Medicines is a UKMi resource (produced with input from the SMC and the
AWMSG) that provides advance information to NHS organisations on around 30–50
new medicines (and new licensed indi-cations) with market launches anticipated
in the next 18–24 months. It is a signposting document (as opposed to an
evaluation document) giving brief details of likely indications, pharmacology,
available clinical trial results and estimates of potential uptake.
The content of
Prescribing Outlook, New Medicines is not comprehen-sive but focuses on
medicines with potential for significant clinical, financial or service
delivery implications for the NHS. A number of criteria are applied to those
medicines considered for inclusion to help prioritise those likely to have the
largest impact, including whether:
· the drug is expected
to provide significant improvement in disease management
· the drug is ‘first
in class’
· there are limited
other drug/non-drug alternatives
· the cost of the
medicine will be high
· the target
population is large
· there are likely to
be significant service implications, e.g. there may be increased monitoring
requirements
· the medicine or
disease area is considered an NHS priority
· the medicine has
significant additional indications in the advanced pipeline stage
· the medicine is in
the EU licensing process
· there is likely to
be significant media interest.
For more detailed
evaluations of medicines in development, the NPC, in collaboration with UKMi,
publishes evaluations of key new medicines – around six per year – under the On
the Horizon title. Recently the collabo-ration has started to publish
‘blog-style’ On the Horizon commentaries evaluating the design and outcome of
individual clinical trials of medicines likely to be launched in the next year
or so.
The pressure on
prescribing budgets is not solely associated with new medicines: a significant
pressure arises from the issue of new national guidance, such as that produced
by NICE, or the impact of major clinical trials on clinical practice. UKMi
produces a further resource, Prescribing Outlook, National Developments, which
aims to provide the NHS with advanced warning of such prescribing pressures. It
includes information on relevant documents, target populations, potential
financial implications for the NHS and issues that need to be considered by
commissioners and providers of services.
A further UKMi
planning resource is Prescribing Outlook – Cost Calculator, which is based on
the other two Prescribing Outlook documents. It is an Excel spreadsheet that
allows crude calculations of potential costs of prescribing changes for a local
population, facilitating budgetary prediction. Prescribing Outlook is published
annually in the autumn to meet the NHS planning timetable for the following
year. The value of Prescribing Outlook to NHS organisations as a
horizon-scanning tool has been highlighted by the Audit Commission.
Early horizon scanning
While the resources
discussed above support NHS organisations’ short-term planning needs, there is
also a need for a longer-term view of new medicines that are in the development
pipeline. UKMi maintains a database, NewDrugsOnline (NDO) that tracks drugs
from the phase IIb/III develop-ment stage to product launch. NDO is used to
inform the content of Prescribing Outlook; it also drives the work plan of
other organisations, such as LNDG.
NDO can be accessed
by NHS staff who have a budgetary planning role relating to medicines. Although
the full content is only available to those who are registered users of the
database, as it contains information which has been provided by pharmaceutical
companies on a confidential basis, much of the public domain information is
made freely available through the NHS website portal, NHS Evidence.
NDO contains around
1000 monographs relating to medicines in devel-opment, including significant
licence extensions to existing therapies. It is updated daily with information
from a number of sources, including medical news feeds, journals and company
and licensing authority press releases. The content is also informed through
direct one-to-one meetings with all major pharmaceutical companies in the UK.
The database can be used to meet a variety of needs, including allowing
planners to keep up to date with devel-opments in between annual publications
of Prescribing Outlook. In addition, it is used, for example, by clinical
pharmacists to update directorate staff on developments in their speciality, by
medicine management committees for forward-planning purposes, by specialist
commissioners to identify medicines that may fall within their jurisdiction and
by those involved in clinical trial work. The advanced search facility of the
database allows complex reports to be run to fulfil individual requirements.
The Next Stage
Review recognised the importance of horizon scanning in facilitating an
increase in the rate of appropriate uptake of new medicines by the NHS. Under
the auspices of the Ministerial Industry Long-term Leadership Strategy Group,
the Department of Health and the Association of the British Pharmaceutical
Industry proposed the development of a national horizon-scanning database,
populated with information by the pharmaceutical industry, to support directly
organisations, such as UKMi, that have an NHS horizon-scanning role. The
database, known as UK Pharmascan, was launched in mid-2010. UKMi will be using
this database to improve and advance its own early planning resources for new
medicines.
Specialist information services
There are a significant
number of specialised medicines-related subjects that are frequently referred
to due to their nature, which MI pharmacists therefore have to address
routinely. Some of these subject areas are of a clinical nature, for example
drug use in pregnancy, whilst some are of a pharmaceutical nature, for example
medicines that are latex-free. As they are in subject areas that are frequently
encountered, a network of MI services providing a spe-cialist information
advisory service in some of these areas has been established over the past 25
years (summarised in Table 8.6).
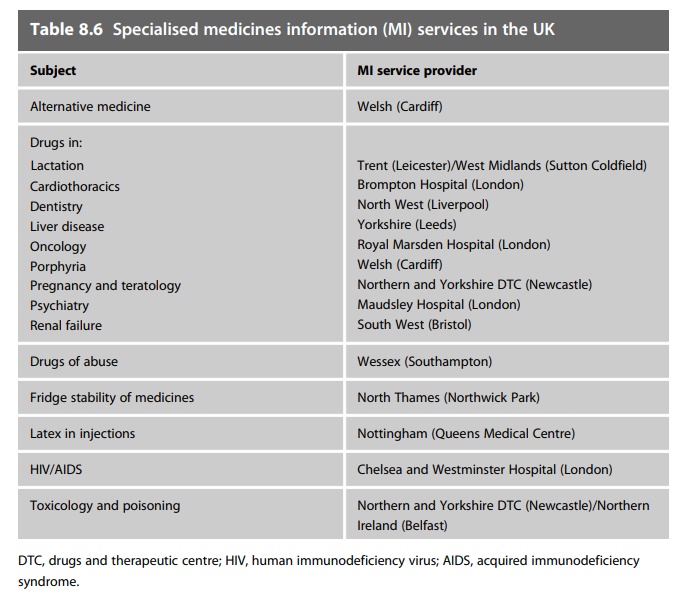
This network provides several distinct advantages. Firstly, in clinical areas, it enables the establishment of a depth of understanding and expert-ise that would not be feasible in every MI service. This includes more comprehensive coverage of the evidence base for that subject, and estab-lishment of clinical ‘expert’ contacts to augment the information provided. Secondly, for non-clinical subjects, it enables the compilation of compre-hensive databases of specialised product information, often with the col-laboration of the pharmaceutical industry. In all cases it provides either a single contact source for users or a single back-up source for MI services, and it reduces a substantial amount of work duplication. The quality of the information is also significantly enhanced. These specialised services were originally based on regional MI centres, but have expanded to encompass the specialties able to be provided from local MI centres based in specialist hospitals or in hospitals providing a high-level specialty in its clinical portfolio. The availability of some of these services is restricted to MI pharmacists only, whilst others have a more open availability and may be wholly or partly web-enabled.
National electronic Library for Medicines
NeLM
(www.nelm.nhs.uk) is the largest MI portal for healthcare profes-sionals in the
UK NHS. It aims to promote the safe, effective and efficient use of medicines.
This free service has been in operation since 1998 and is updated daily. The
site has a wide range of information products, including news, evidence-based
reviews on drugs and drug therapy and material to support health promotion. It
also provides a facility for sharing practice. Much of the current content is
provided by UKMi.
Current awareness
services offered by NeLM include a personalised daily e-mail newsletter and a
wide range of rich site summary (RSS) feeds on specific topics. The Medicines
A–Z facility uses codes from the NHS Dictionary of Medicines and Devices (dmþd:
http://www.dmd.nhs.uk/) to present on a single page evidence about an
individual medicine which is held in NeLM, integrated with links to further information
in external sources, including the British National Formulary (BNF), the
electronic Medicines Compendium (eMC) and the National Injectable Medicines
Guide (Medusa: password-protected NHS website).
NeLM also
incorporates selected content from the former Pharm-line bibliographic database
to create an area of NeLM dealing with the broad area of medicines management
and pharmacy practice.
The current NeLM is
the result of a series of transformations as the site has been redeveloped to
provide a comprehensive medicines knowledge base. It will store and link to a
wide range of MI products procured or produced by the NHS. The vision is to
build a system for the integration of knowledge for utilisation at local health
community level. Some NeLM con-tent can already be retrieved using the NHS
Evidence search interface.
Other NHS or related
organisations that produce medicines information which is available through the
NeLM portal include:
·
NPC
·
NICE
·
Medicines Healthcare product and Regulatory Authority
·
Department of Health
·
SMC.
In addition,
information from professional bodies such as the Royal Pharmaceutical Society
and the British Medical Association and independent organisations such as the
eMC is also available via NeLM.
The objectives of
the NeLM are:
·
to develop a ‘one-stop’ platform from which users can easily
find medicines information that matters in a simple and coherent manner
·
to establish a mechanism for aggregating medicines knowledge
to support electronic prescribing and similar applications
·
to produce local medicines knowledge bases for health
communities to support local prescribers. These will reflect local practice
while integrating with national resources of MI.
Information resources
MI services require
access to a wide range of published information sources to fulfil all the
activities outlined. Many activities may require access only to standard
reference books or systems that provide relatively static, unchanging
information. However, as the role of the MI pharmacist has developed, the
information sources required have become more evidence-based and current, often
with use of the primary literature as the main source of information. Whilst
textbooks will remain a valuable source of information, their limita-tions
(cost, currency, completeness) restrict their importance and are chang-ing the
way in which information and clinical evidence are accessed and utilised. Most
core, standard information sources are now available electron-ically, commonly
via the internet, which makes them not only more accessible but increasingly
more cost-effective to use.
MI services in the
UK have established a minimum information resources standard, that is, a
collection of core information resources that all MI services must either hold
in house or to which they must have immediate and unrestricted access. The
resources contained in this minimum standard are continually reviewed and
updated for new editions, new titles and obsoles-cence. The current information
resource standard contains reference and textbooks, journals and electronic
databases and is common for all MI ser-vices. In addition, larger local MI
centres, regional MI services and specialist MI services will have a
requirement for a wider range of resources which will partly be defined by national
standards and partly by service need. One basic premise of information resource
use is that only the most recent editions of resources should be used.
Out-of-date resources could lead to unreliable and erroneous information that
could result in harm to a patient, or even a legal challenge.
There are also a
large number of important resources that are freely accessible and considered
useful to NHS MI services, including free paper resources (for example, BNF,
Drug Tariff) and freely accessible websites via the internet or via national
library arrangements. Full current lists of recom-mended and important free
information resources can be found at www. ukmi.nhs.uk.
Expanding internet
and intranet publishing will increasingly make many of the remaining paper-based
resources more readily available, often in a form that is ‘free to end-user’,
although this will inevitably include current users of MI services. The
implications of this shift in the balance of information access and utilisation
will have to be taken into account by service providers, includ-ing MI
services.
In addition to
published resources, MI services will also use in-house collections of
commercial and published literature, in-house databases and collections of past
outputs (past enquiries, frequently asked questions, bul-letins, reports, and
so on) to augment information access.
Utilising information technology
MI services are
heavily dependent on all aspects of information technology and electronic
communications, both to acquire and to disseminate informa-tion. Online
resources have been outlined and provide the dominant platform for
evidence-based information on medicines, medicine management and pharmacy
practice. Electronic and web-based internet and/or intranet appli-cations are predicted
to continue to develop and expand in the foreseeable future. This presents both
threats and opportunities to MI services. The main threat, as already
described, is that information is becoming more directly and readily available,
and in more useful formats and outputs, to the end-user, including healthcare
professionals and patients. The quality, accuracy and validity of many of these
‘accessible’ sources are, however, open to question, with no guidance to users
either to increase their awareness of the inherent issues or to help them make
judgements about quality, appro-priateness and contextual interpretation. The
opportunities, however, are for MI services to produce and present quality
information that will assist patients and healthcare professionals to make
valid judgements on the use of medicines that maximise safety and
effectiveness. The positive aspects of these information platforms must also be
fully utilised, both to communicate within the MI network and to disseminate
information to patients and healthcare staff, through increasingly popular
features including, at the time of writing, RSS news feeds, blogs and podcasts,
personal customisation of online resources, web conferencing and webinars,
online learning and assessment.
Developments in the
functionality and accessibility of hardware and gadgets will also influence how
MI will adapt to its users by making evidence-based information and decision
support more rapidly available to the user for application at the point of
care. Similarly, electronic pre-scribing will have an impact on how MI services
engage with prescribing and patient care. Some of the basic decision-making
processes of electronic prescribing will be embedded in the decision support
and clinical knowledge systems inbuilt into electronic prescribing packages.
Whilst this may remove some of the basic clinical problem-solving tasks
currently undertaken by clinical and MI pharmacists, the need for
patient-specific customised infor-mation and solving more complex problems will
be a crucial role for the MI pharmacist. Validation of the information
contained in e-prescribing sys-tems will also need to be undertaken to ensure
prescribing and patient care are not compromised.
Established methods
of electronic information communication, such as websites and e-mail, are now
taken for granted in day-to-day practice. Over the past decade the intuitive
nature of their use, their immediate access to information and users, and their
ready availability in most work and home settings have radically and
fundamentally changed the way in which MI services function.
·
MI services in the UK have used new technologies and
electronic commu-nication to enhance service access, delivery and processes,
although the poten-tial for further developments, as outlined, is enormous,
largely constrained only by the need for investment. Apart from the NeLM,
developments in MI include the following:
·
MiDatabank: this is a Windows software application that
enables MI services to record, manage and store their enquiries. MiDatabank was
developed specifically for the UKMi network, and has now been adopted as the UK
national standard. It is used by over 200 MI centres processing more than 500
000 enquiries per year. It is also being used internationally. One of its crucial
features is to provide an audit trail of the complete enquiry-answering
process. One of the future objectives of MiDatabank is to facilitate sharing
enquiry-related research and answers between MI centres nationally and
internationally through web-based applications.
·
The national MI website, www.ukmi.nhs.uk, provides a
one-stop resource for MI in the UK, containing a wide range of information,
including training and research resources and a single source of all strategic,
clinical governance and operational policies and MI guidelines. It also gives
access to all the major clinical outputs of the national UKMi network. The
national MI website is augmented by a UK-wide e-mail discussion group, MI-UK,
which supports information-sharing.
Workforce and training
As with most areas
of professional practice, the most important resource of MI services is its
staff. MI services are normally managed by experienced clinical pharmacists
with specialist training to develop the additional skills, competencies and knowledge
required. Training in MI skills and techniques begins at the preregistration
stage, with most pharmacy graduates undergoing specific MI training before
registration; this also meets the core statutory requirements for pharmacists’
competencies. After registration this is aug-mented by further training, both
formally and through placements in estab-lished MI centres. Developing clinical
and problem-solving skills supports MI-related skill development, useful in
ward and more formal MI service settings.
Pharmacists
undertaking more formal work in an MI service are then normally exposed to more
formal MI training on a national basis. In the UK, a structured training
programme is in place, starting with a national introductory MI training course
that introduces the pharmacist to the range of skills and activities relevant
to an MI specialist. Entry to this course requires a basic level of
predetermined competencies acquired through structured work experience. This
level of training aims to enable the trainee to:
·
understand and apply the skills, knowledge and resources to
provide clinically oriented MI
·
apply the basic principles of searching electronic sources
of information, in particular Medline, Embase and the internet
·
know the strengths and weaknesses of the key MI databases
·
apply basic statistical tests to clinical trial data
·
identify the key components of clinical trial design and
apply these to critical appraisal of the literature
·
know the necessary verbal communication skills required to
deliver an effective MI service
·
identify legal and ethical problems that may be encountered
when providing MI
·
understand the principles of clinical governance, and in
particular risk management and quality, and apply these to the provision of MI.
This training is
supported by the availability of a national MI workbook. More advanced training
to develop skills,knowledge and effective use of resources is then provided to
suit the needs of the individual and the service.
The whole training
strategy is supported by a national competency frame-work for MI, introduced in
2001. The aim of this is to identify the compe-tencies that individuals working
in MI either have, or need to develop, in order to perform their work
effectively now and in the future. The framework is used to:
·
facilitate continuing professional development at an
individual level
·
help managers and MI pharmacists identify ongoing training
and development needs
·
provide a framework to support local recruitment and
appraisal processes.
As competencies for
all aspects of clinical pharmacy practice are developed, MI competencies will
be aligned and integrated into more general, national competency frameworks, at
both standard and advanced practice levels, to reflect the MI provider role of
all clinical pharmacists.
Although pharmacists
have been the principal professional component of the MI workforce in the past,
other professional groups are now being developed, and actively deployed, in MI
services to support and provide some of its activities. In particular,
experienced pharmacy technicians are now in the service, as they are in many
other areas of clinical pharmacy practice. Appropriate technicians, once
identified, are trained through a rigorously controlled programme which
includes core training, in-house work, super-vised work experience, development
of a personal work evidence portfolio, continuous assessment, a probationary
period and subsequent accreditation as an MI technician. Accredited technicians
can then assume some respon-sibilities within the enquiry-answering process.
Currently, there are seven common enquiry types, for which MI technicians can
have a substantive responsibility, although the final responsibility for the
overall process remains with the MI pharmacist. These are:
·
tablet and capsule identification
·
availability of medicines
·
formulation and stability (excluding parenteral)
·
interactions
·
adverse effects
·
complementary medicines
·
travel medicine.
Routine activity
within these seven designated subject areas is covered by the accreditation
process. However, MI technicians can be involved in other enquiry types and
other MI activities as long as adequate training has been undertaken and risk
management issues assessed.
The future
development of MI technicians’ activities and responsibilities will be an
important feature of future service development which will take account of
skill mix, recruitment and clinical competence issues. Other pro-fessional
groups, including information scientists, life science graduates, librarians and
others, may also have roles to fulfil in future MI services which are yet to be
defined.
Related Topics
