The Innate Immune System
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Immunology
Innate defence against the passage of potentially pathogenic microorganisms across epidermal and mucosal barriers involves a range of non-specific mechanisms.
THE INNATE IMMUNE SYSTEM
1) Innate Barriers At Epidermal And Mucosal Surfaces
Innate defence against the passage of potentially pathogenic
microorganisms across epidermal and mucosal barriers involves a range of
non-specific mechanisms. Commensal microorganisms living on mucosal surfaces
and on membranes such as skin and conjunctiva constitute one such mechanism).
These commensals are, under normal circumstances, non-pathogenic, and help
prevent colonization by pathogenic strains. There are also a number of physical
and chemical barriers against microbial entry, including the flow of fluid
secretions from tear ducts, the urogenital tract and the skin. Many of these
secretions possess bacteriostatic or bactericidal activity due to their low pH
or the presence of hydrolytic enzymes such as lysozyme (a peptidoglycan
hydrolase). Similarly, the mucus barrier covering mucosal surfaces such as the
epithelium of the lung serves as a false binding platform for microorganisms,
preventing them from interacting with the underlying host cells. In the normal
state the hydrated mucus barrier is efficiently cleared under the driving force
of beating cilia. The serious lung infections seen in cystic fibrosis arise
because patients are unable to clear the bacteria-laden dehydrated mucus effectively.
2) Innate Defence Once Epidermal Or Mucosal Barriers Have Been Compromised
The function of the innate defence system against microorganisms that
have penetrated into interstitial tissues and the vascular compartment relies
largely on the processes of phagocytosis and of activation of the alternative
complement pathway. However, the functions of the innate system when exposed to
microbial infection are also critical in the recruitment and activation of
cells of the adaptive immune response.
The main cells mediating phagocytosis are the mononuclear phagocytic
cells and granulocyte cell populations; of the latter, neutrophils are particularly
important. For such cells to function, they must possess receptors to sense
signals from their environment. In executing their effector functions they need
to secrete a range of molecules that will recruit or activate other immune
cells to a site of infection.
Before consideration of the process of phagocytosis, an overview of the
mononuclear phagocytic cell and granulocyte cell populations is useful.
a) Mononuclear phagocytic cells
The mononuclear phagocytic cells include monocytes and macrophages.
Monocytes make up approximately 5% of the circulating blood leucocyte
population and are short-lived cells (circulating in blood for ≤ 8 hours), but
migrate into tissue to give rise to tissue macrophages. The macrophages
constitute a long-lived, widely distributed heterogeneous population of cell
types which bear different names within different tissues, such as the
migrating Kupffer cell within the liver or the fixed mesangial cell within the
kidney glomerulus.
The mononuclear phagocytic cells secrete a wide range of molecules too
numerous to list in full here. However, these secretions include:
•
Molecules which can break down or
permeabilize microbial membranes and thereby mediate extracellular killing of
microorganisms, e.g. enzymes (lysozyme or cathepsin G), bactericidal reactive
oxygen species and cationic proteins.
•
Cytokines which can provide innate protective antiviral (e.g. interferon
(IFN)-α or-β) and antitumour (e.g. TNF-α) activity against other host cells. A
group of cytokines termed chemokines can
also serve to chemoattract other leucocytes into an area of ongoing infection
or inflammation, for example IL-8 which attracts neutrophils. Yet another group
of cytokines has proinflammatory actions (e.g. IL-1 and TNF-α) which, among
other outcomes, leads to activation of endothelial and leucocyte cells
promoting increased leucocyte extravasation into tissues and, in the case of
IL-1, activation of T-lymphocyte populations.
•
Bioactive lipids (e.g. thromboxanes, prostaglandins and leukotrienes), which
further promote the inflammatory response through actions to increase capillary
vasodilation and permeability.
•
The mononuclear phagocytic cells also
possess numerous receptors that interact with their environment. These cells
possess, among others:
•
Receptors for chemotaxis toward microorganisms,
e.g. receptors for secreted bacterial peptides such as formylmethionyl peptide.
•
Receptors for complement proteins that
serve as leucocyte activators (e.g. C3a and C5a; see section 2.2.4) or
complement proteins that serve to coat (opsonize) microorganisms (e.g. C3b). An
opsonized microbial surface more readily adheres to a phagocyte membrane, with
the opsonin triggering enhanced activity of the phagocyte itself.
•
Receptors for promoting adherence, such
as lectin receptors interacting with carbohydrate moieties on the surface of
the microorganism, or receptors for Fc domains (non-antigen-recognition
domains) of antibodies which opsonize microorganisms (e.g. the receptor for the
Fc domain of IgG is Fcg), or integrin receptors for cell-cell adhesion (e.g.
promoting interaction between a macrophage and T-lymphocyte).
•
Receptors for cytokines including those
involved in macrophage activation (e.g.IFN.-γ) or limiting macrophage mobility
(e.g. macrophage inhibitory factor, MIF) and hence increasing cell retention at
a site of infection.
b)
Granulocyte cell populations
The granulocyte cell populations include the neutrophils, basophils and
eosinophils. The short-lived (2–3 days) neutrophil is the most abundant
granulocyte (comprising > 90% of all circulating blood granulocytes) and is
the most important in terms of phagocytosis; indeed, this is the main function
of the neutrophil. The receptors and secretions of the neutrophil are similar
to those of the macrophage, although notably the neutrophil does not present
antigen via MHC class II proteins (see later). The neutrophil is recruited to
sites of tissue infection or inflammation by a neutrophil-specific chemotactic
factor (IL-8) and is also chemoattracted and activated by some of the same
factors described for mononuclear phagocytic cells, including complement
protein C3a, bacterial formylmethionyl peptides and leukotrienes. Like
macrophages, neutrophils undergo a respiratory burst and are very effective
generators of reactive oxygen species.
Eosinophils are poor phagocytic cells and have a specialized role in the
extracellular killing of parasites such as helminths, which cannot be
physically phagocytosed. Basophils are non-phagocytic cells.
c) Phagocytosis
Macrophages and neutrophils in
particular demonstrate a high capacity for the physical engulfment of particles
such as microorganisms or microbial fragments from their immediate
extracellular environment. This process (Figure 9.2) is made up of a number of steps:
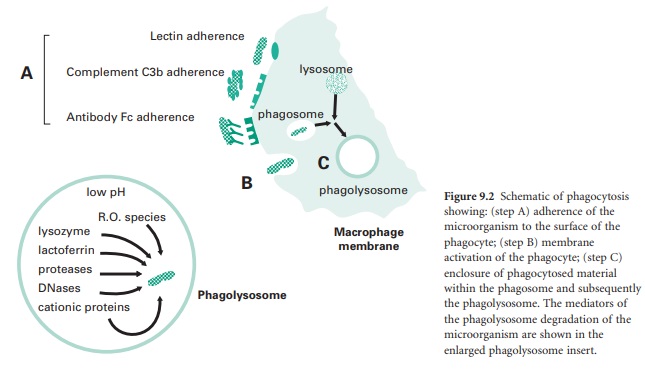
•
Chemotaxis of the phagocyte toward the
microorganism through signals arising from the microorganism itself (e.g.
formylmethionyl peptide), signals arising from complement proteins (e.g. C3a
and C5a) generated as part of the activation of the alternative complement
pathway (see section 2.2.4), or signals due to release of inflammatory factors
(e.g. leukotrienes) secreted by other leucocyte cells situated at the site of
an infection.
•
Adherence of the microorganism to the
surface of the phagocyte (step A in Figure 9.2), involving adhesion through lectin
receptors present on the surface of the phagocyte which interact with
carbohydrate moieties on the surface of the microorganism; adhesion through
complement C3b receptors present on the surface of the phagocyte interacting
with C3b molecules that have opsonized the surface of the microorganism; and
adhesion through Fc receptors which interact with the Fc domain of antibodies
that have opsonized the surface of a microorganism.
•
Membrane activation of the phagocyte
actin-myosin contractile network to extend pseudopodia around the attached
microorganism (step B in Figure 9.2). Membrane activation will also lead to the
generation of a ‘respiratory burst’ by the phagocyte which involves an increase
in the activity of the phagocyte membrane NADPH oxidase which converts
molecular oxygen into bactericidal reactive oxygen species such as superoxide
anion (• O−), hydrogen peroxide (H2O2), and in
particular hydroxyl radicals (•O H) and halogenated oxygen metabolites (HOCl−).
•
The enclosure of phagocytosed material,
initially within a membranous vesicle termed a phagosome.
Here, cationic proteins such as defensins and reactive oxygen species begin
microbial membrane degradation. This is followed within minutes by fusion of
the phagosome with a lysosome to form a phagolysosome, whose contents are at an
acidic pH of about 5 which is optimal for the continued active breakdown of
microbial structural components (step C in Figure 9.2).
d) Alternative complement pathway
The alternative complement pathway
fulfils a critical role in innate immune defence. The complement system
comprises at least 20 different serum proteins; many are known by the letter C
and a number, e.g. C3. Many of the complement proteins are zymogens, i.e. proenzymes requiring proteolytic
cleavage to be enzymically active themselves; some are regulatory in function.
The cleavage products of complement proteins are distinguished from their
precursor by the suffix ‘a ’ or ‘b ’, e.g. C3a and C3b, with the suffix ‘b’
generally denoting the larger fragment that stays associated with a microbial
membrane, and the suffix ‘a’ generally denoting the smaller fragment that
diffuses away. The activation of the complement pathway occurs in a cascade
sequence, with amplification occurring at each stage, such that each individual
enzyme molecule activated at one stage generates multiple activated molecules
at the next. In the ‘resting’ state, in the absence of infection, the
complement proteins are inactive or have only a low level of spontaneous
activation. The cascade is tightly regulated by both soluble and membrane-bound
associated proteins. The regulation of the complement pathway prevents
inappropriate activation of the cascade (i.e. when no infection is present) and
also minimizes damage to host cells during an appropriate complement response
to a microbial infection. Complement activation is normally localized to the
site(s) of infection.
There are three main biological functions of the alternative complement
pathway.
• Opsonization of microbial
membranes. This involves the covalent binding of complement proteins
to the surface of microbial membranes. This opsonization or coating by
complement proteins promotes adherence of the opsonized microbial component(s)
to the cell membranes of phagocytic cells. The complement protein C3b is a
potent opsonin.
• Activation of leucocytes.
This involves complement proteins acting on leucocytes, either at the site of
infection or at some distance away, with the result of raising the level of
functioning of the leucocytes in immune defence. For example, C3a is a potent
leucocyte chemoattractant and also an activator of the respiratory burst.
• Lysis of the target cell
membrane. This involves a collection of complement proteins
associating on the surface of a microbial membrane to form a membrane attack
complex (MAC), which leads to the formation of membrane pores and, ultimately,
microbial cell lysis.
Figure 9.3 shows a highly schematized view of the
activation cascade for the alternative complement pathway on a microbial
membrane surface. The activation steps in the alternative pathway are also
shown in Figure 9.7, which contrasts with the activation steps
in the classical complement pathway involving antibody.
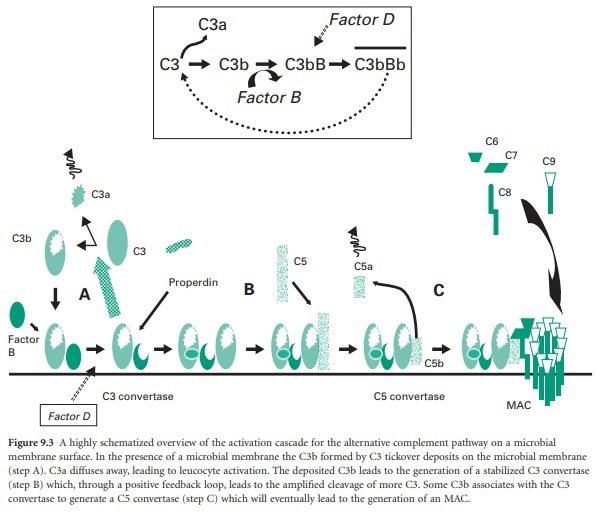
Figure 9.3 A highly schematized overview of the
activation cascade for the alternative complement pathway on a microbial
membrane surface. In the presence of a microbial membrane the C3b formed by C3
tickover deposits on the microbial membrane (step A). C3a diffuses away,
leading to leucocyte activation. The deposited C3b leads to the generation of a
stabilized C3 convertase (step B) which, through a positive feedback loop,
leads to the amplified cleavage of more C3. Some C3b associates with the C3
convertase to generate a C5 convertase (step C) which will eventually lead to
the generation of an MAC.
The pivotal protein in the alternative pathway is C3 (195 kDa). Under
normal circumstances (in the absence of infection) C3 is cleaved very slowly
through reaction with water or trace amounts of proteolytic enzyme to give C3b
and C3a. The C3b formed is susceptible to nucleophilic attack by water and is
rapidly inactivated to give iC3b. The C3a is not generated in sufficient
amounts to lead to leucocyte activation and is rapidly inactivated. This normal
low-level cleavage of the C3 molecule is termed ‘C3 tickover’ and it provides
low levels of starting material, i.e. C3b, which will be required for full
activation of the alternative complement pathway in the case of a microbial
infection.
In the presence of a microbial membrane
the C3b formed by C3 tickover will be susceptible to nucleophilic attack by
hydroxyl or amine groups on the membrane surface, leading to the covalent
attachment of C3b to the membrane (step A in Figure 9.3). Once C3b has attached to the membrane,
factor B can bind to form a molecule termed C3bB. This complex is stabilized by
a soluble protein called properdin. Factor D then enzymically cleaves the bound
factor B to generate a molecule termed C3bBb which is the C3 convertase of the
alternative pathway (Figure 9.3 inset).
This newly generated stable C3 convertase enzymically cleaves C3 to
generate further C3b and C3a molecules, leading to leucocyte activation (by
C3a) and greater deposition of C3b on the microbial membrane and hence further
generation of C3 convertase molecules. In effect the microbial membrane has
activated a positive feedback loop with cleavage of C3 to generate high amounts
of C3b and C3a molecules.
The deposited C3b not only leads to the
formation of the C3 convertase but also coats the microbial membrane as an
opsonin and so promotes binding to phagocyte cell membranes. Some of the
deposited C3b associates with the newly formed C3 convertase to generate a
complex termed C3bBb3b, which is the C5 convertase of the alternative pathway
(step B in Figure 9.3). This C5 convertase binds the complement
protein C5 and cleaves it into C5a (a leucocyte activator) and C5b (an
opsonin). The C5b remains associated with the membrane and acts as a plat-form
for the sequential binding of complement proteins C6, C7, C8 (step C in Figure 9.3). The α-chain of the C8 molecule penetrates
into the microbial membrane and mediates conformational changes in the incoming
C9 molecules such that C9 becomes amphipathic (simultaneously
containing hydrophilic and hydrophobic groups). In this form it is capable of
insertion through the microbial membrane where it mediates a polymerization
process that gives the MAC. The MAC generates transmembrane channels within the
microbial membrane with the osmotic pressure of the cell leading to an influx
of water and eventual microbial cell lysis.
Differences between host cell membranes and microbial cell membranes
mean that the cascade is only activated in the prresence of microorganisms, so
C3 tickover cannot give rise to full activation of the alternative pathway in
the absence of microbial membrane. Stable deposition of a functional C3
convertase only occurs on the microbial cell surface. The differences that
exist include, for example:
• lipopolysaccharide or peptidoglycan on microbial membranes that
promote the binding of C3b
• the high sialic acid content of host cell membranes that promotes the
dissociation of any C3 convertase formed on host surfaces
• the presence of specific host cell membrane proteins that also serve a
key regulatory function.
Decay activating factor (DAF) or complement receptor type 1 (CR1) are
host cell membrane proteins that serve to competitively block the binding of
factor B with C3b and hence inhibit formation of a C3 convertase; they also
promote disassembly of any C3 convertase formed. Membrane cofactor protein (MCP)
and CR1 are further host cell membrane proteins that promote the displacement
of factor B from its binding with C3b. Host cell membranes also possess a
protein, CD59, which prevents the unfolding of C9—a required step for membrane
insertion to form an effective MAC.
Related Topics
