Activated Complex
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Mechanisms of Organic Reactions
Both of the considerations given above are related because the activated complex is an unstable and high-energy collection of atoms which has bonds and electron distributions distorted relative to those in both the reactants and products.
ACTIVATED COMPLEX
Both
of the considerations given above are related because the activated complex is
an unstable and high-energy collection of atoms which has bonds and electron
distributions distorted relative to those in both the reactants and products.
The structure of the activated complex is determined by the bonding and
structural changes that must occur in order for the reactants to be converted
to products. Moreover the more the structure of the activated complex is
distorted from nor-mal geometries and bond distances, the higher is its energy.
Consequently more energy must be added to the system to distort the reactants
to the structure of the activated complex.
As
a structural bridge between reactants and products, the activated complex has
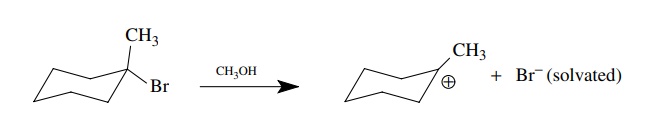
structural features of both. For example, consider a very simple reaction, the
ionization of a tertiary bromide to a carbocation and a bromide ion in
methanol. The reactant is a fully covalent molecule with a complete, yet
polarized, bond between the tertiary carbon and the bromine atom.
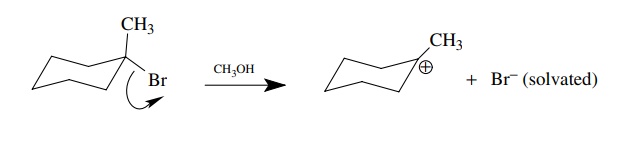
The
products contain a trivalent, positively charged carbon atom surrounded by a
solvent shell and a bromide ion which carries a negative charge and is
surrounded and hydrogen bonded to a shell of solvent molecules. The distance
between the carbon and the bromine atom in the products is much greater than in
the reactants. The bond between carbon and bromine is broken as the bromide
moves away from the carbon and the pair of bonding electrons ends up in the
valence shell of the bromide ion. We can depict this reaction using
curved-arrow notation which tracks electron movement.
However,
between reactants and products is an activated complex which must have
structural features common to both. Qualitatively the activated complex (‡) can
be pictured as a structure in which the bromine has started to move away from
carbon, lengthening the C–Br bond.
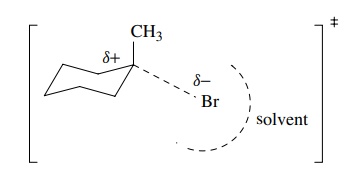
It
has begun to develop a partial negative charge since the bonded pair of
electrons is even more displaced onto the bromine. For the same reason, the
tertiary carbon has begun to develop partial positive charge. The bond
connecting carbon and bromine is longer and weaker than in the reactant, but
there is still some bonding energy between carbon and bromine that is
completely absent in the products. In addition, due to the greater amount of
negative charge on bromine in the activated complex than in the reactant, the
solvent starts to organize around the bromine, but the interaction is not
nearly so strong as in the bromide ion product. The solvation of the
carbocation product is much weaker than the solvation of the bromide in a
hydroxylic solvent and is often disregarded; however, to the extent that the
carbocation product is solvated, the solvent will begin to solvate the
partially charged carbon in the activated complex.
The
foregoing has been a rather microscopic description of the activated com-plex
(perhaps too detailed), but the idea is quite clear. At whatever level the
activated complex is described, it must contain structural features of both the
reactants and the products it connects; that is, it can only be described in
terms of the stable structures of reactants and products.
This
then is the dilemma. The activated complex occurs at the transition state and
has a vanishingly short lifetime (∼1 bond vibration or ∼10−13 sec) yet its
structure and energy must be described and changes in its structure and energy
must be evaluated if we are to compare different reactants in a predictive way.
Related Topics
