Activation Energy
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Mechanisms of Organic Reactions
The first and most important fact about any chemical reaction or any elementary step in a chemical process is that there is an energy barrier separating the products from the reactants.
ACTIVATION ENERGY
The
first and most important fact about any chemical reaction or any elementary
step in a chemical process is that there is an energy barrier separating the
products from the reactants (Figure 5.1). For the reactants to be converted to
products, the
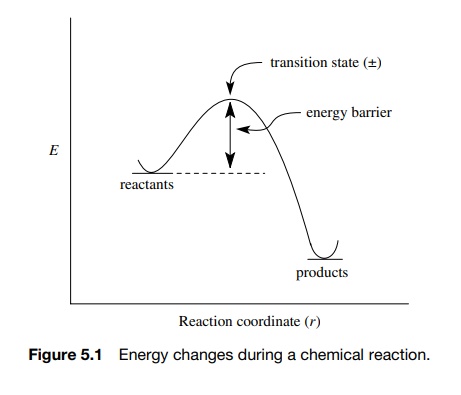
Then and only then can the reactants change into
products by passing over the barrier and relaxing into the product state. The
energy barrier is of prime importance because (a) the height of the barrier
determines how fast reactants can be converted to products at a given
temperature and (b) at the maximum height of the energy barrier, which is
called the transition state, there is a collection of atoms, called the activated
complex, that is the structural bridge between
reactants and products.
Related Topics
