Depiction of Mechanism
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Curved-Arrow Notation
Use of curved-arrow notation to depict the mechanisms of organic reactions requires that appropriate mechanistic principles be superimposed on the correct use of curved arrows to denote movement of electrons.
DEPICTION OF MECHANISM
Use
of curved-arrow notation to depict the mechanisms of organic reactions requires
that appropriate mechanistic principles be superimposed on the correct use of
curved arrows to denote movement of electrons. The mechanism of a reaction is
the stepwise process by which reactants are converted to products, and
generally each step involves bond making and/or bond breaking that can readily
be depicted by curved-arrow notation.
Simple
substitution reactions are shown in curved-arrow notation as
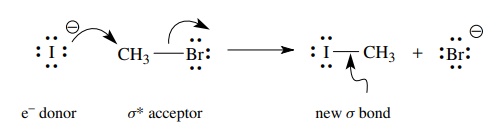
In
this example a nucleophile donates electrons to the electrophile, in this case
a carbon with a leaving group attached, to produce a new σ bond. As the new σ bond is formed, the bond to the leaving group
breaks and the substitution of one group for another is completed. The iodide
nucleophile has unshared pairs of electrons which are donated. The σ ∗ orbital of the C–Br
bond is the acceptor orbital. In any donor – acceptor interaction which leads
to a chemical change, identification of both the donor and acceptor is very
crucial in predicting what change will occur and determining how the change
might occur.
Addition
of Grignard reagents to carbonyl groups involves donation of the electrons of
an electron-rich carbon – metal σ
bond to the π∗ orbital of the car-bonyl group. As shown below, carbon –
carbon bond formation is accompanied by carbon – oxygen π bond cleavage, oxygen – metal bond formation, and a
cor-responding change in geometry from trigonal to tetrahedral.
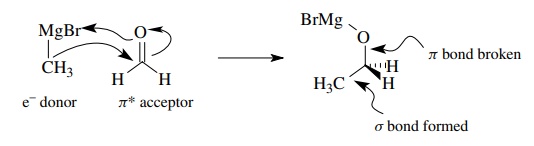
Ring
opening of epoxides by alkoxides is used to emphasize that charge must be
conserved during each mechanistic step. Because the reactants as written
(neglecting spectator ions) have a net negative charge, the products must have a net negative charge.
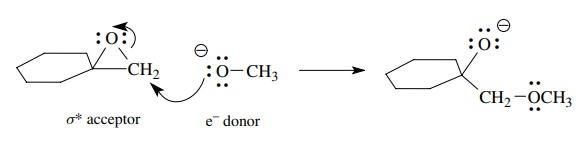
The
conservation of charge is a fundamental law for all processes, such as the
addition of nucleophiles to π systems
or acid – base reactions. The first step of the basic hydrolysis of nitriles
has the hydroxide ion adding to the π
bond of the nitrile. For the purposes of mechanistic discussion, the hydroxide
is shown without its counterion and the net charge on the reactant side of the
equation is −1. Consequently, the
product of this first step (and each subsequent step) must also have a net negative charge.
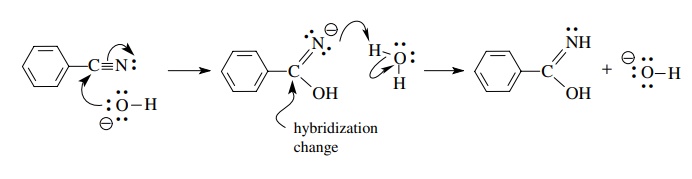
In
aqueous solution, proton transfer to the first formed intermediate is very rapid. However, again for
illustrating the stepwise changes that must occur on the way from reactants to
products using curved-arrow notation, these steps are shown independently.
Similarly,
the production of enolates from carbonyl compounds involves base removal of a
proton from the α position. The
enolate is negatively charged and has delocalized electrons.
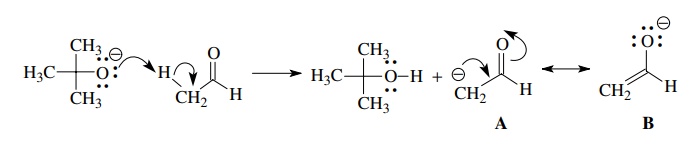
Although
this process can be written to give a single canonical form A, it must be realized that the enolate
is a delocalized species and resonance forms A and B can be generated
as discussed previously using curved-arrow notation. This is not a mechanistic
step since the delocalized product is a resonance hybrid of A and B— that is A is not
converted to B, but rather the
curved arrows merely indicate the
changes in electron distribution that must be used to describe the canonical
form B.
These
previous examples are reactions where the electron donor (nucleophile) supplies
the “electronic push” to accomplish bond breaking. Many nucleophiles, either
neutral or anionic, have lone pairs of electrons that are easily donated. They
can be donated to even weak electron acceptors (electrophiles).
There
are other reactions, also easily describable by electron movement (curved
arrows), in which π electrons are
donated. In such reactions the π
electrons are bonded electrons and hence the π-donor nucleophile is a weak electron donor. Consequently, a much
stronger electron acceptor (stronger electrophile) is required for the
“electronic pull” for electron donation to occur successfully. However, such
descriptions are simply a matter of semantics because curved-arrow notation
only shows changes in electrons, it does not indicate driving force. For a
donor – acceptor interaction to occur productively, there has to be an energetic
driving force for the process, and the energy levels of the donor and acceptor
must be matched so that electron movement from the donor to the acceptor can
occur.
For
example, the protonation of a double bond has a proton as the electrophile and
the π bond as the electron donor.
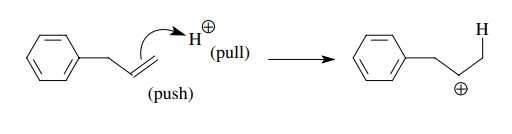
When
bonded electrons are donated, it is important to remember that one of the
bonded atoms which shared that pair is now left without a valence octet. That
is, removal of a bonded pair must result in a sextet atom. Such is the case
after protonation of a double bond as seen above. Because of the instability of
a sextet electronic configuration, several strategies are available to
stabilize it.
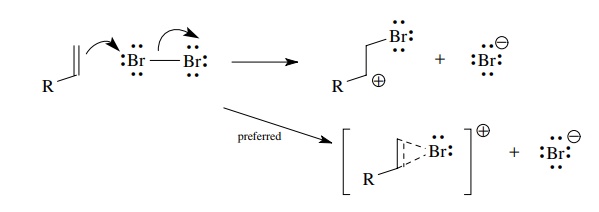
Neighboring
atoms having unshared pairs of electrons can undergo bridg-ing interaction with
the cationic center to give structures in which all atoms have valence octets.
An archetypical example is the bromination of olefins. Electrophilic addition
of bromine to the double bond is predicted to give an α-bromocarbocation.
However, formation of the bridged bromonium ion avoids a sextet configuration
of carbon and thus is formed preferentially. Bridging inter-actions occur when
a carbocation is generated vicinal to substituents such as –OR, –Cl, –F, –SR, –NR2,
and so on, all of which have lone pairs capable of bridging interactions.
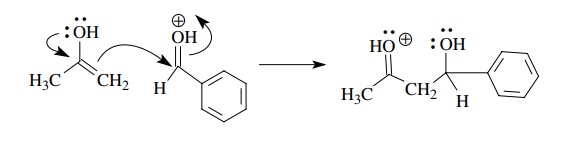
Resonance
stabilization can also make π-electron
donation much more effec-tive by avoiding the formation of a sextet
carbocation. Lone-pair donation from the oxygen of enol derivatives is very
important to the good donor ability of these compounds. The resulting oxonium
ion has all valence octets (although positively charged) and is thus stabilized
over sextet canonical forms.
Resonance
stabilization is important in electrophilic aromatic substitution as well.
While each of the canonical forms of the Wheland intermediate has a sextet
carbon atom, the charge is distributed over the remaining five atoms of the
ring by resonance and is thus greatly stabilized.
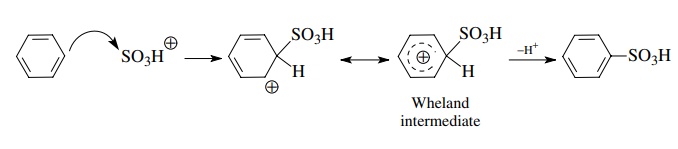
The
reactions of nucleophiles with electrophiles also relates to the overall
oxidative change of a reaction. As is expected, nucleophilic atoms which are
more electronegative than carbon are not reductants and usually give no change
in oxidation state, for example,
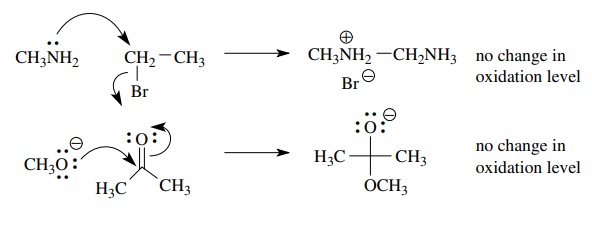
Conversely
nucleophiles which are carbanion or hydride equivalents are reductants,
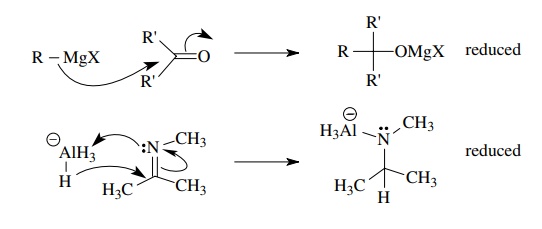
Carbocation
or proton electrophiles give no change in oxidation level whereas electrophiles
which are electronegative elements (Br2, Cl2, NBS,
peracids, etc.) are oxidants,
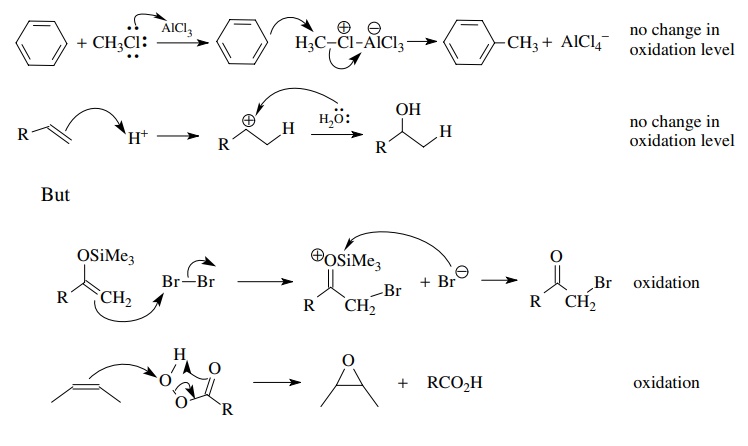
Besides
intermolecular reactions, curved-arrow notation is also useful in indicating
bonding changes in intramolecular reactions and rearrangement. For example,
Cope-type rearrangements are seen to involve changes in three pairs of bonded
electrons.
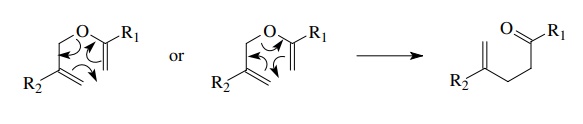
The
arrows can be written in either directional sense since these reactions are concerted
rearrangements with all bond making – bond breaking taking place at the same
time. This example emphasizes the fact that curved-arrow notation is merely an
electron bookkeeping method.
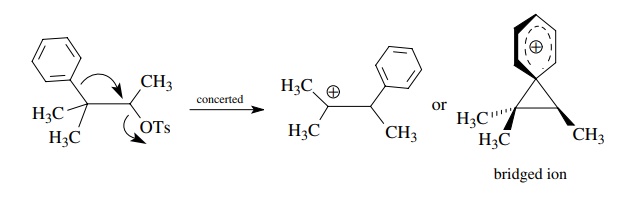
Cationic
rearrangements are also handled easily by keeping track of where electron pairs
come from and where they go. For example, the neophyl-type rearrangement below
leads to skeletally rearranged products.
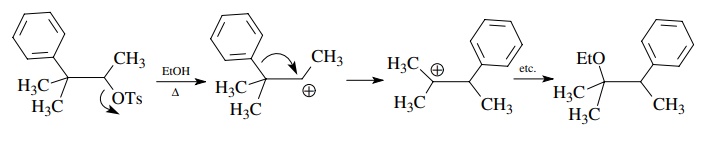
The
curved-arrow notation clearly shows the electron flow needed to effect the
rearrangement. What curved-arrow notation does not show is the timing of these
events — that is, whether loss of a leaving group precedes or is concerted with
1,2-phenyl migration or if a bridged ion is an intermediate. Such
considerations, if known, can be included in more detailed mechanistic
sequences.
With
these considerations, then, the steps one goes through to use electron movement
to generate a possible reaction mechanism are as follows:
1.
Write a balanced equation for the reaction. While spectator ions may be
neglected, it is imperative to write correct Lewis structures for reactants and
products. This step is very important but often neglected.
2. Note the connectivity changes that occur, changes in
oxidation level that occur, and the reagents or reactant types necessary for
the conversion.
3. Write a stepwise process for the reaction using curved
arrows to account for bonding changes. The use of curved arrows for electron
movement should be guided by bond polarities, donor – acceptor properties,
electroneg-ativities, and structural factors and should result in a reasonable
series of bonding changes from reactant to product.
4. Evaluate intermediates for stability and valence. If they
fit normal chemical expectations, then the mechanism is potentially correct.
There may be other mechanisms operating or the timing of individual steps
(synchronous, con-certed, etc.) may be different, but the above process can be
used to generate them as well.
Thus
we see that, used properly, curved-arrow notation for electron movement is
indispensable to the organic chemist as a way to depict chemical change in
com-plex molecules. Furthermore, it can be extended to include a method for
showing the mechanism if the ground rules are understood and followed
carefully.
Related Topics
