Activation of the Classical Complement Pathway
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Immunology
A complement cascade similar to that of the alternative pathway can be activated through specific antibody–antigen interactions. The antibodies that activate the classical complement pathway are IgM and IgG.
ACTIVATION OF THE CLASSICAL COMPLEMENT
PATHWAY
A complement cascade similar to that of
the alternative pathway can be activated through specific antibody–antigen
interactions. The antibodies that activate the classical complement pathway are
IgM and IgG.
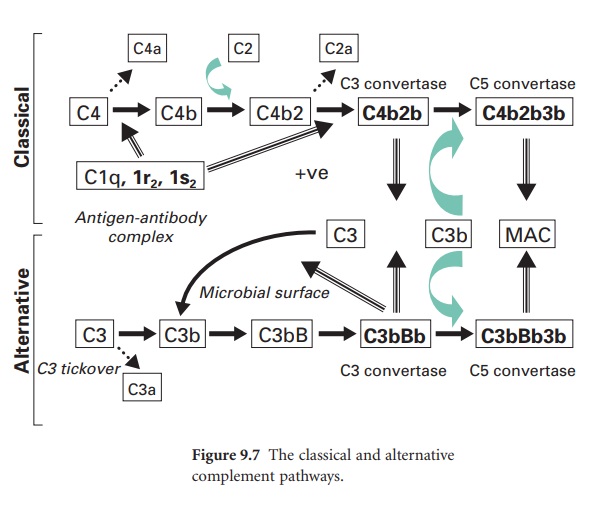
Key steps in the activation of the
classical pathway are shown in Figure 9.7, where this pathway is also compared
to the alternative pathway. In the classical pathway the initiating step is the
specific binding of IgG or IgM to antigen. Once this occurs, a complement
protein termed C1 (which comprises a single C1q subunit, two C1r subunits and
two C1s subunits) binds to adjacent Fc domains in the antibody–antigen complex.
This binding of C1 activates the catalytic activity of the C1r subunits, and in
turn the C1s subunits. The activated C1s subunits cleave C4 into C4b and C4a;
the latter can diffuse away and serve as a leucocyte activator. The C4b
covalently associates with the antibody–antigen complex on the surface of a
microbial membrane and can serve as an opsonin. A further complement protein,
C2, binds to this membrane complex to give C4b2. The C1s subunit then
enzymically cleaves the bound C2a to generate on the membrane a new complex termed
C4b2b, which is the C3 convertase of the classical pathway. (In some texts the
C2a is referred to as the larger subunit remaining with the membrane while C2b
is the smaller subunit that diffuses away.)
This C3 convertase molecule is distinct
from that within the alternative pathway, but it is from this point onwards
that parallels can be drawn between the two cascades.
The host proteins that serve key
regulatory functions within the alternative pathway (DAF, CR1 factor I, CD59)
also serve similar functions within the classical pathway. However, in contrast
to the alternative pathway the activation step in the classical pathway
requires specific antibody–antigen interactions. In this context the C1 protein
can only become catalytically active when it is bound to at least two adjacent
Fc domains. In the case of the IgG and IgM molecules the Fc domains will only
align adjacent to each other when the corresponding Fab domains bind antigen.
Further, when C1 is free in the circulation it is bound to a protein termed C1
inhibitor (C1-INH) which prevents any possible activation of C1 in the absence
of antibody. Once C1 binds to adjacent Fc domains within an antibody–antigen
complex C1-INH is displaced.
The functions of the classical complement
pathway are similar to those described for the alternative pathway, i.e.
opsonization, leucocyte activation and membrane lysis of target cells. The
classical pathway can additionally lead to complement protein deposition on
insoluble antibody– antigen immune complexes circulating within blood, and in
doing so promote the clearance of such potentially harmful complexes by Kupffer
cells of the liver. The presence of two complement pathways provides for rapid
(alternative) and specific (classical) activation of a key defence mechanism,
and offers greater protection against the development of microbial resistance
mechanisms.
Related Topics
