Processing of Proteins to Allow Peptide Presentation by MHC Molecules
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Immunology
Peptide epitope presented by MHC I is derived from the processing of proteins (e.g. a viral protein) synthesized within the actual cell that eventually will present the peptide to cytotoxic T-lymphocytes.
PROCESSING OF PROTEINS TO ALLOW PEPTIDE
PRESENTATION BY MHC MOLECULES
Peptide epitope presented by MHC I is derived from the processing of proteins (e.g. a viral protein) synthesized within the actual cell that eventually will present the peptide to cytotoxic T-lymphocytes. The MHC I molecule is composed of two polypeptide chains, an α chain which has α1, α2 and α3 domains, and a second polypeptide termed β2-microglobulin. The α1 and β2 domains form a peptide-binding cleft which can accommodate peptides up to 11 amino acids in length. Figure 9.9a shows the processing of a protein into peptide fragments for presentation by MHC I. The synthesized protein (indicated by an asterisk) is present in the cytoplasm of the cell and is degraded by a subcellular organelle termed a proteasome. The derived peptide fragments are actively transported, via a TAP peptide transporter, into the lumen of the endoplasmic reticulum (ER) where they fit within the binding clefts of MHC I molecules. From the ER the MHC I with bound peptide is transported to the trans-Golgi network (TGN), from which it is transported via endosomes to the plasma membrane where the MHC I molecule with bound peptide is accessible to surveillance by cytotoxic CD8+ T-lymphocytes.
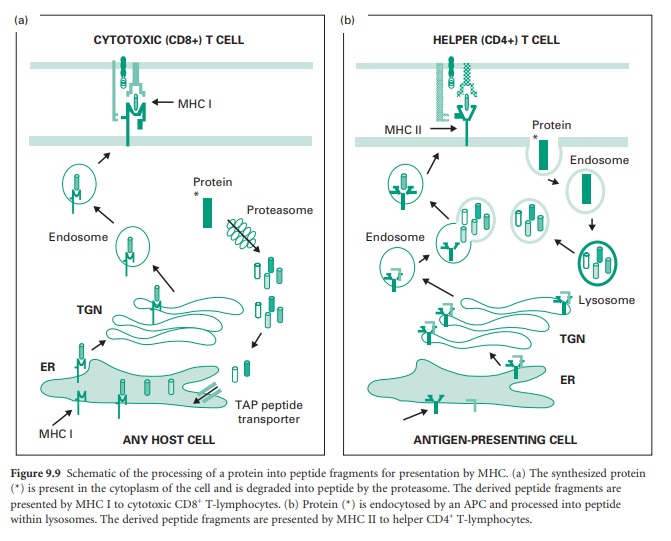
Peptide epitopes presented by MHC II are
derived from proteins present within the extracellular fluid and are presented
to helper T-lymphocytes by APCs. The MHC II molecule is composed of two
polypeptide chains, an α-chain which has α1 and α2 domains, and a β-chain which
has β1 and β2 domains. The α1 and β1 domains form a peptide-binding cleft that
can accommodate peptides up to 20 amino acids in length. Figure 9.9b shows the
processing of a protein into peptide fragments for presentation by MHC II. In
this case the protein (indicated by an asterisk) is internalized from the
extracellular fluid by the APC and restricted to an endosomal compartment
without access to the APC’s cytoplasm. The endosome delivers the protein to a
lysosome compartment which degrades the protein into peptide fragments, after
which the peptide fragments are returned to an endosome compartment. In the
lumen of the ER the MHC II molecule becomes associated with another protein
termed an invariant chain which blocks access of peptides to the binding cleft
of the MHC II molecule; the MHC II–invariant chain complex is transferred to
the TGN and then to an endosomal compartment. At this point the endosomes that
contain the processed peptide and the MHC II molecules merge, and the invariant
chain disintegrates, allowing peptides access to the MHC II-binding cleft. The
MHC II molecules with bound peptide are transported to the plasma membrane
where they are accessible to surveillance by helper CD4+ T-lymphocytes.
Related Topics
