Artemisinin-Based Combination Therapy (ACT)
| Home | | Pharmacology |Chapter: Essential pharmacology : Antimalarial Drugs
Noting that use of antimalarial drugs singly has failed to curtail the prevalence of malaria globally, particularly due to emergence of chloroquine-resistant followed by multidrug-resistant P. falciparum, the WHO has recommended that acute uncomplicated resistant falciparum malaria should be treated only by combining one of the artemisinin compounds with another effective erythrocytic schizontocide.
ARTEMISININ-BASED COMBINATION THERAPY (ACT)
Noting that use of
antimalarial drugs singly has failed to curtail the prevalence of malaria
globally, particularly due to emergence of chloroquine-resistant followed by
multidrug-resistant P. falciparum, the WHO has recommended that
acute uncomplicated resistant
falciparum malaria should be treated only by combining one of the artemisinin
compounds with another effective erythrocytic schizontocide. In choosing the
companion drug, the most important consideration is its elimination t½
(governing stay in the body), because effective concentrations in blood must be
maintained for at least 3 asexual cycles of the parasite, i.e. 6 days, to
exhaust the parasite burden. Therefore, short t½ drugs have to be given for 7
days, while longer acting drugs can be given for 1–3 days. However, long t½
drugs allow sub-inhibitory concentrations to persist in the blood facilitating
selection of resistant mutants. Combining a short t½ drug with a long t½ drug
in the conventional 3 day regimen runs the risk of de facto monotherapy after the short t½ drug is eliminated. This
risk is minimized by choosing a short t½ drug that reduces the parasite load
rapidly and drastically. Artemisinin compounds fillin this requirement, as they
rapidly kill > 95% plasmodia. They leave only a small biomass of the
parasites to be eliminated by the long t½ drug, reducing the chances of
selecting resistant mutants. Advantages of ACT over other antimalarials are:
· Rapid clinical and
parasitological cure.
· High cure rates
(>95%) and low recrudescence rate.
· Absence of parasite
resistance (the components prevent development of resistance to each other).
· Good tolerability
profile.
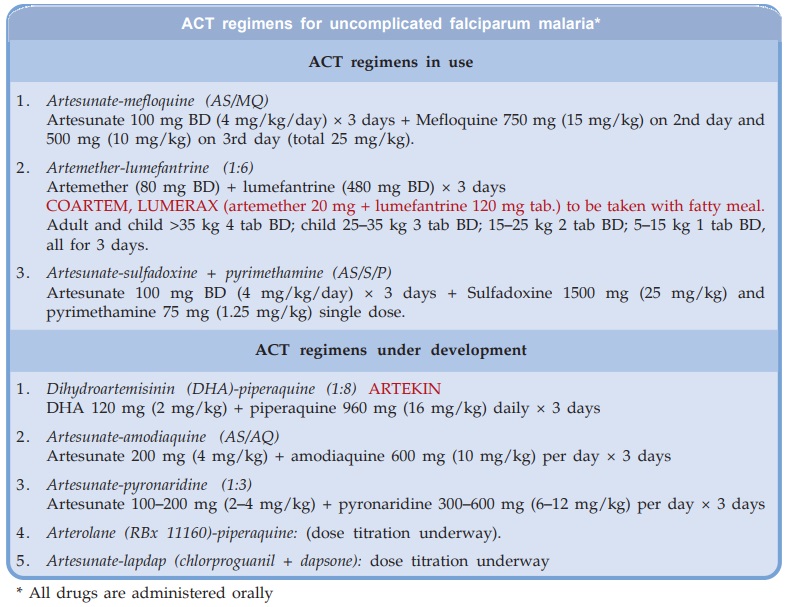
The ACT regimens for
oral treatment of uncomplicated resistant falciparum malaria that are already
in use in India, and those being clinically evaluated are given in the box. They
are not to be used in severe or complicated malaria, for which parenteral drugs
are needed.
Artesunatesulfadoxine + pyrimethamine (AS/S/P)
This ACT has been
adopted as the first line drug for
falciparum malaria in chloroquineresistant areas under the ‘National
antimalaria drug policy’ of India, and has replaced chloroquine in 73 districts.
However, this does not imply that it is the most effective/best ACT, because it
is not effective against multidrugresistant strains which are nonresponsive to
S/P. Moreover, no comparative evaluation of this regimen against AS/MQ or
artemether/ lumefantrine, etc. has been done to establish the relative
efficacy/tolerability.
Artesunate-mefloquine (AS/MQ)
It has been extensively used in Thailand, Myanmar and
several other countries; found to be highly effective, well tolerated and is
now the first line treatment for uncomplicated falciparum malaria in Southeast
Asia. Many areas in the far East already have mefloquine-resistant P. falciparum. By combining with artesunate,
further spread of mefloquine-resistance has been prevented.
Artemether-lumefantrine
Lumefantrine is an orally active, high efficacy, long-acting
erythrocytic schizontocide, related chemically and in mechanism of action to
halofantrine and mefloquine. It acts in the food vacuole of plasmodia to
inhibit haeme polymerization.
Additionally, nucleic acid and protein synthesis of the parasite
is affected. Like the others, vivax hypnozoites are not affected. Lumefantrine
is highly lipophilic; absorption starts after 2 hours of ingestion and peaks at
6–8 hours; action is slower than chloroquine. Plasma protein binding is 99%,
and it is metabolized predominantly by CYP3A4. It inhibits the isoenzyme CYP2D6.
Terminal t½ is 2–3 days, which is prolonged to 4–6 days in malaria patients.
Lumefantrine has been used only in combination with artemether,
and is the only ACT currently available as fixed dose combination tablets. The two
components protect each other from plasmodial resistance. As such, no clinically
relevant resistance has developed so far. Clinical efficacy is high (>95%
cure rate) and comparable to artesunatemefloquine. It is active even in multidrug-resistant
areas, including mefloquine-resistant. While artemether quickly reduces
parasite biomass and resolves symptoms, lumefantrine prevents recrudescence.
Gametocytes are rapidly killed, cutting down transmission.
Lumefantrine-artemether is administered with food, preferably
fatty food or milk, which markedly enhances lumefantrine (and to some extent
artemether) absorption. It is generally well tolerated; side effects
are—headache, dizziness, sleep disturbances, abdominal pain, arthralgia,
pruritus and rash. Some studies indicate that it is better tolerated than
artesunate mefloquine. Lumefantrine-artemether should not be given with drugs
metabolized by CYP2D6 (metoprolol, neuroleptics, tricyclic antidepressants,
etc), or with drugs which prolong QTc interval. It is contraindicated in first
trimester of pregnancy and during breastfeeding.
Dihydroartemisinin (DHA) Piperaquine
Piperaquine is a bisquinoline congener of chloroquine
developed in China as a high efficacy long-acting (t½ 2–3 weeks) erythrocytic
schizontocide with a slower onset of action. The mechanism of action is similar
to chloroquine, and it is equally active against sensitive, but more active
against chloroquine resistant P.
falciparum. In 1978, piperaquine replaced chloroquine in China, where it
has been extensively used for mass prophylaxis as well as treatment of malaria.
Piperaquine has been
combined with DHA in a dose ratio of 8:1 (ARTEKIN) and extensively
evaluated in multidrug resistant areas of Cambodia, Thailand, Vietnam, etc.
with high success rate. In clinical trials, efficacy of DHApiperaquine fixed
dose combination has been found comparable to artemether-lumefantrine or
artesunate-mefloquine. Safety profile of DHApiperaquine is good and it is well
tolerated even by children. However, dizziness, vomiting and other g.i. symptoms
are common; rashes are rare. It is undergoing clinical trials in India.
Artesunate-Pyronaridine
Pyronaridine is a
watersoluble Mannich base erythrocytic schizontocide with high efficacy and
mechanism of action similar to chloroquine, that has been used in China for
> 30 years. It is active against both chloroquine-sensitive and resistant P. falciparum
and other malarial species. The onset of action is slower and duration long. It is concentrated in RBCs and
metabolized with a terminal t½ of 7 days. Weak analgesic, antipyretic actions
are produced at higher doses.
Clinical efficacy of
artesunate-pyronaridine fixed dose combination (dose ratio 1:3) has been tested
in falciparum malaria in China and Africa with >95% success and no
recrudescence in 28 days. Multi-drugresistant P. falciparum and P. vivax
also respond. Phase II clinical trials have been completed in India. Artesunate-pyronaridine
is well tolerated. Side effects noted are abdominal pain, vomiting, headache,
dizziness, loss of appetite, palpitation and transient ECG changes, but no
serious reactions have occurred.
Artesunate-Amodiaquine (AS/AQ)
Though the extent of cross resistance between chloroquine
and amodiaquine is uncertain, several trials in Africa have found amodiaquine
to cure 41–79% P. falciparum infections
in chloroquine resistant areas. Addition of artesunate improved the cure rate
to 68–85%. Administered as separate tablets, the two drugs have been
effectively used to treat resistant falciparum malaria in Africa. A fixed-dose
combination of the two has been produced and is being tested for efficacy and
tolerability in Africa, Southeast Asia and India. Early results are
encouraging, and it is likely to emerge as a low cost ACT.
Arterolane (RBx 11160)-piperaquine
Arterolane is an orally active synthetic trioxolane congener
of artemisinin with rapid and potent erythrocytic schizontocidal action on
plasmodia, including multidrug-resistant P.
falciparum. Like artemisinins, it is short-acting (t½ 2–4 hours) and has shown efficacy in
treating falciparum malaria. Trials are being conducted in India and it is
likely to be combined with piperaquine to yield a low cost and well tolerated
ACT.
Artesunate-chlorproguanil-dapsone
Since chlorproguanildapsone
combination (Lapdap) is already in use for treatment of malaria in several
countries, a fixed dose combination by addition of artesunate is being
clinically tried as an alternative low cost ACT.
