Atoms, Molecules, and Chemical Bonds
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Chemical Basics of Life
The composition of matter and changes in its com-position are the focuses of the study of chemistry.
Atoms,
Molecules, and Chemical Bonds
The composition of matter and
changes in its com-position are the focuses of the study of chemistry. If you
understand chemistry, your understanding of anatomy and physiology improves.
Chemical changes within cells influence body functions and the status of the
body’s structures. Chemicals of the body include water, proteins,
carbohydrates, lipids, nucleic acids, and salts as well as foods, drinks, and
medications.
Elements are fundamental substances that com-pose matter. Copper,
iron, gold, silver, aluminum, carbon, hydrogen, and oxygen are all examples of
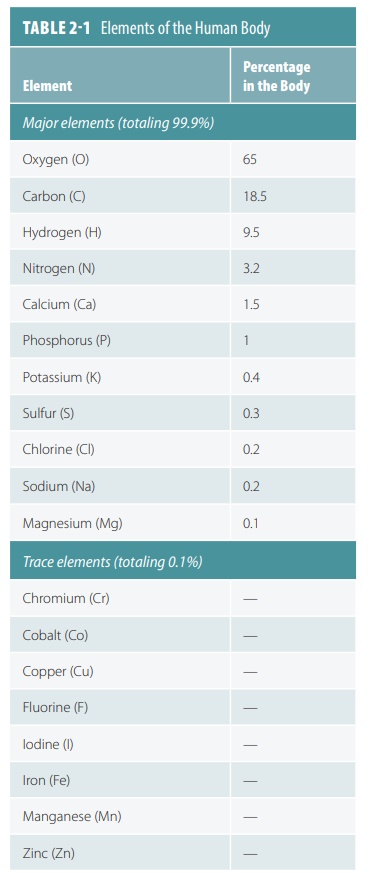
ele-ments. Most living organisms need about 20 elements to survive. TABLE 2-1 lists the major and trace elements required by the human body.
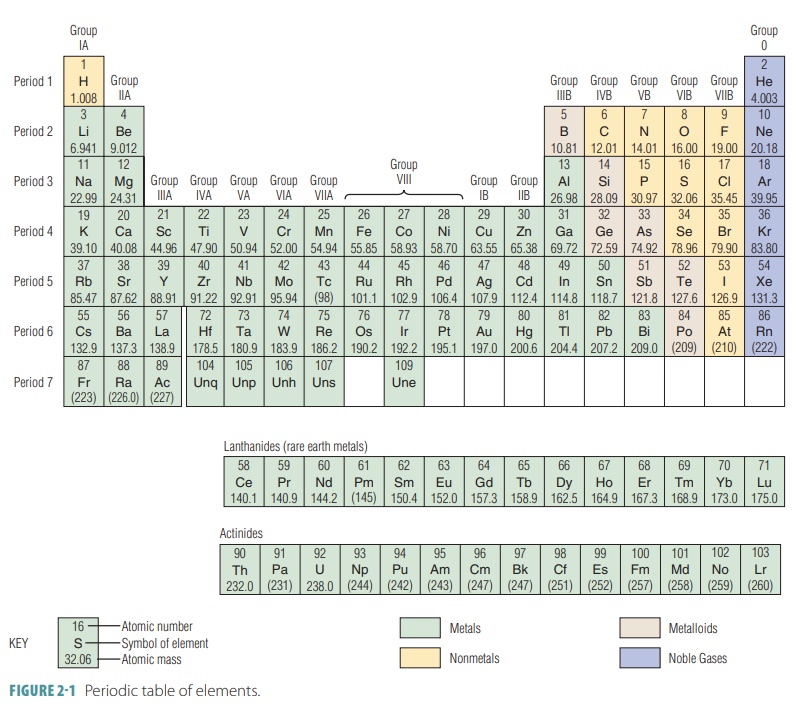
The periodic table of ele-mentsis a
tabular arrangement of the chemical ele-ments, and organized on the basis of
atomic numbers, electron configurations, and recurring chemical prop-erties.
The elements are presented in order of increas-ing atomic number, which is the
number of protons in the nucleus (FIGURE 2-1).
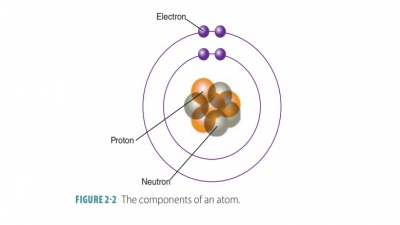
Atoms are tiny particles that compose elements,and are the
smallest complete units of an element that retain its properties and vary in
size, weight, and interaction with other atoms.
The characteristics of living and nonliving objects result from the atoms they
contain and how those atoms combine and interact. Thus, by forming chemical
bonds, atoms can combine with other atoms that are not similar to them