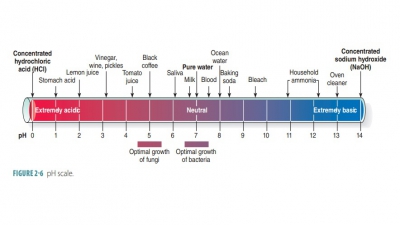
Inorganic Substances
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Chemical Basics of Life
Inorganic substances in body cells include water, salts, and acids/bases.
Inorganic
Substances
Inorganic substances in body
cells include water, salts, and acids/bases.
Water
The most abundant compound in the
human body is water, accounting for nearly two-thirds of the body weight. Any
substance that dissolves in water is called a solute. Because solutes dissolved in water are more likely to react
with each other as they break down into smaller particles, most metabolic
reactions occur in water. There are five major properties of water:
■■ Cushioning: Protection of
certain organs from physical trauma (e.g., cerebrospinal fluid that cushions
the brain)
■■ High heat capacity: Ability to
absorb and release large amounts of heat before water itself actually changes
temperature to any large degree; this pre-vents sudden temperature changes from
external factors such as exposure to sun or wind or from internal conditions
that quickly release heat suchas vigorous muscle activity; in the blood, water
redistributes heat among tissues, maintaining homeostasis
■■ High vaporization heat: Water
changes from liquid to gas (water vapor), which requires large amounts of heat
to be absorbed in order to break hydrogen bonds that keep water molecules
together; this is valuable as part of sweating—since mostly water evaporates
from the skin, the body is efficiently cooled
■■ Polar solvent properties:
Water is referred to as the universal solvent, since nearly all chemical
reactions in the body require its solvent proper-ties. As a result,
biochemistry is called wet chem-istry. Biological molecules only react
chemically when they are in a solution. Water molecules are polar, and their
slightly negative ends are oriented toward the positive ends of solutes, The
reverse is also true. Solute molecules are attracted and then surrounded. This
is why acids, bases, other small reactive molecules, and ionic compounds
dissoci-ate in water. Their ions separate from each other and scatter
throughout the water, forming true solutions.
Water also forms hydration layers
of its mol-ecules around large charged molecules such as proteins. Hydration
layers shield the mol-ecules from other nearby charged substances, preventing
them from settling out of the solu-tion. These protein-water mixtures are known
as biological colloids. Examples include blood plasma and cerebrospinal fluid.
The solvent properties of water
make it the body’s major transport system. Metabolic wastes, nutrients, and
respiratory gases are carried to be dissolved in the blood plasma.
Many metabolic wastes are
excreted as urine. Lubricants such as mucus use water to dis-solve other
substances.
■■ Reactivity: For many chemical
reactions, water is an important reactant; an example is when foods are broken
down to their components by adding a water molecule to each bond.
Salts
Salts are compounds of oppositely
charged ions that are abundant in tissue fluids. A salt is an ionic com-pound
that contains cations other than hydrogen ions and anions other than the
hydroxyl ion. When salts are dissolved in water, they dissociate into their
component ions. One example is when sodium sulfate (Na2SO4) dissociates into
two sodium ions and one SO42– ion. It dissociates easily since its ions are
alreadyformed. Water then easily overcomes the attraction between the
oppositely charged ions.
All ions are electrolytes, which
conduct electrical currents when in solution. Electrolytes release ions in
water. As they dissolve in water, the negative and posi-tive ends of water
molecules cause ions to separate and interact with water molecules instead of
each other. The resulting solution contains electrically charged particles
(ions) that conduct electricity. Groups of atoms that have an overall charge,
such as sulfate, are known as polyatomic ions.
In the body, common salts include
sodium chlo-ride (NaCl), calcium carbonate (CaCO3), and potas-sium chloride
(KCl). The most plentiful salts are the calcium phosphates, which are utilized
to harden the bones and teeth. The ionized form of salts is used for vital body
functions. Salt ions are important for transporting substances to and from the
cells, muscle contractions, and nerve impulse conduction. Ionic iron makes up
part of the hemoglobin molecules transporting oxygen inside red blood cells.
Certain enzymes require zinc and copper ions. Other import-ant functions of
elements from body salts include:
■■ Calcium: Found as a salt in
bones and teeth, itsionic form is needed for blood clotting, conduction of
nerve impulses, and muscle contraction
■■ Chlorine: Its ion, chloride,
is the most abundantanion in extracellular fluids
■■ Iron: A component of
hemoglobin and someenzymes
■■ Phosphorus: Part of calcium
phosphate salts inbones and teeth, also in nucleic acids, and is a partof
adenosine triphosphate (ATP)
■■ Potassium: Its ion is the
major positive cation incells; vital for conduction nerve impulses andmuscle
contraction
■■ Sodium: Its ion is the major
positive ion in extra-cellular fluids; important for conduction of
nerveimpulses, muscle contraction, and water balance.
1. Distinguish
between organic and inorganic chemicals.
2. Define
the term “biochemistry” and list its other descriptive title.
3. What
are the five major properties of water?