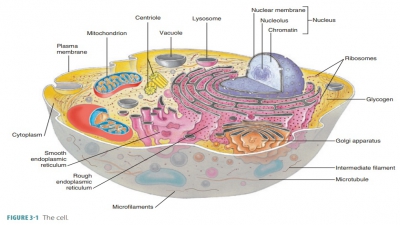
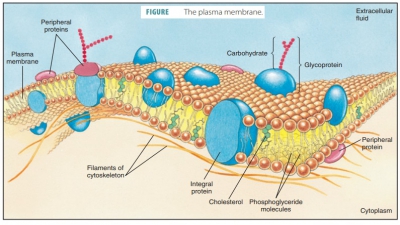
Summary
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Chemical Basics of Life
Chemistry describes the composition of substances and how chemicals react with each other.
Summary
Chemistry describes the
composition of substances and how chemicals react with each other. The human
body is made up of chemicals. Matter is composed of elements, some of which
occur in a pure form. There are various states of matter, including gaseous,
liquid, and solid states. Energy is different from matter, taking up space,
having no mass, and only being measured by how it affects matter. Energy is the
capacity to put matter into motion or to perform “work.”
Many elements are combined with
other elements. They are composed of atoms, which are the smallest complete
units that still have the properties of the elements they form. Atoms of
different elements have characteristic sizes, weights, and ways of interacting.
An atom consists of one or more electrons surround-ing a nucleus, which
contains one or more protons and usually one or more neutrons. Electrons are
negative, protons are positive, and neutrons are uncharged. When atoms combine,
they gain, lose, or share elec-trons. Atoms of the same element may bond to
form amolecule of that element. Compounds that release ions when they dissolve
in water are known as electrolytes. A molecule is any chemical structure that
consists of atoms held together by covalent bonds. Compounds are chemically
pure, with identical molecules.
Mixtures are substances
containing two or more components that are physically intermixed, and include
colloids, solutions, and suspensions. Chemical bonds are energy exchanges
between electrons of reacting atoms, and include ionic, covalent, and hydrogen
bonds. Ionic bonds form between ions. Covalent bonds are formed when electrons
are shared, producing molecules in which the shared electrons are located in a
single orbital common to both atoms. When the positive hydrogen end of a polar
molecule is attracted to the negative nitrogen or oxygen end of another polar
molecule, the attraction is called a hydrogen bond. A chemical reaction occurs
when a chemical bond is formed, broken, or rearranged. The four types of chemical
reactions are synthesis, decomposition, exchange, and reversible reactions.
Catalysts can increase the rate of chemical reactions without becoming
chemically changed themselves or without becoming a part of the product.
Acids are electrolytes that release
hydrogen ions in water. Bases are electrolytes that release ions that bond with
hydrogen ions. Hydrogen ion concentrations can be measured by a value called
pH. Biochemistry (biologic chemistry) is the study of chemical processes within
and relating to living organisms. Organic chemicals are those that contain the
elements carbon and hydrogen and generally oxygen as well. Inorganic chemicals
do not contain these elements. Inorganic substances include water, oxygen,
carbon dioxide, and salts. Organic substances include carbohydrates, lip-ids,
and proteins. Carbohydrates provide much of the energy required by the body’s
cells and help to build cell structures. They contain various types of sugars,
including monosaccharides, disaccharides, and poly-saccharides. Lipids are
insoluble in water, but may dis-solve in other lipids, oils, ether, chloroform,
or alcohol. Fats are the most common type of lipids. Unsaturatefats are safer
than saturated or trans fats. Proteins are the most abundant organic components
of the human body and are the basic structural materials of the body. Nucleic
acids are large organic molecules that carry genetic information or form
structures within cells. They include DNA and RNA.
In the body, enzymes promote
chemical reactions by acting as catalysts to accelerate these reactions without
themselves being permanently changed or consumed. Some enzymes are only made of
protein, whereas others have a protein portion (apoenzyme)and a cofactor. Most
organic cofactors are derived from B complex (or other) vitamins and are known
as coenzymes. ATP pairs with catabolized glucose topower the body. ATP is the
main molecule in cells that transfer energy and provides a type of energy that
all body cells can use immediately.
Related Topics