Passive Cell Mechanisms
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Cells
Passive cell mechanisms include diffusion, osmosis, and filtration.
Passive Cell
Mechanisms
Movements
Through Cell Membranes
The cell membrane controls the
substances that can enter and leave the cell. It does this by using passive and
active mechanisms. Passive mechanisms do not require cellular energy, whereas
active mechanisms do.
Passive Cell Mechanisms
Passive cell mechanisms include
diffusion, osmosis, and filtration.
Diffusion
Diffusion (also known assimple
diffusion) is theprocess by which substances spontaneously move from
regions of higher concentration to regions of lower concentration (the concentration gradient). Molecules and ions in various substances move very quickly,
colliding with many other types of particles. These collisions occur at the rate
of a million times per second. The speed of diffusion is influenced by kinetic
energy, molecular size, and temperature. Once parti-cles have diffused to be
evenly distributed throughout a substance such as water, they have achieved a
stateof equilibrium. Examples of diffusion include ion movement across cell membranes and
neurotransmit-ter movement between nerve cells.
Cells allow substances to diffuse
into or out of them only if the cell membrane is permeable to the substance and if the concentration of a substance
is higher on one side of the membrane than the other (FIGURE 3-13). A
molecule or ion will diffuse through the cell membrane if it is lipid soluble,
assisted by a carrier molecule, or small enough to pass through membrane
channels.
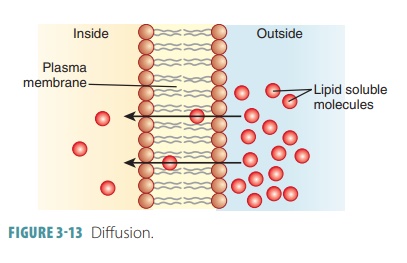
Simple
diffusion is further defined as
unas-sisted diffusion of very small or lipid-soluble particles. Nonpolar and
lipid-soluble substances are diffused through the lipid bilayer. These
substances include carbon dioxide, fat-soluble vitamins, and oxygen. Oxygen
continuously diffuses from the blood into the cells because its concentration
is always higher in the blood than in tissue cells. Oppositely, because carbon
dioxide is in higher concentration within the cells, it diffuses from tissue
cells into the blood.
Some substances cannot pass
through the lipid bilayer of a cell membrane, requiring proteins in the
membrane to assist them. This process is known as facilitated
diffusion, also known asassisted diffu-sion. It is similar to
simple diffusion because it onlymoves molecules from areas of higher
concentration toward areas of lower concentration. Substances that require
facilitated diffusion include certain amino acids, ions, and molecules such as
glucose and other sugars. Facilitated diffusion is a passive transport
pro-cess. Transported substances either bind to protein carriers in the
membrane (and then move across it) or move through water-filled protein
channels.
Therefore, the two types of
facilitated diffusion are called carrier
mediated and channel mediated. Carriers are proteins that are described
astransmem-brane integral. They are
specific for the transportof certain polar molecules or types of molecules
such as sugars and amino acids (which cannot pass through membrane channels
because of their size).
Therefore, the carrier alters its
shape to envelop and later release the transported substance. The carrier
shields the substance as it is moved from the non-polar membrane regions.
Basically, these carrier changes move the binding site from one location on the
membrane to the other.
Just as in simple diffusion,
substances transported by carrier-mediated facilitated diffusion move down
their concentration gradients. For example, glu-cose is usually in higher concentrations
in the blood than in the cells. Therefore, its transport is usually unidirectional—into the cells.
Carrier-mediated trans-port is limited by how many protein carriers
arepres-ent. When all glucose carriers are engaged (saturated), glucose transport occurs at its fastest rate.
Channels are transmembrane proteins that
moveions, water, and other substances through aqueous channels from one side of
a membrane to the other. Because of pore size and amino acid charges in the
lining of the channel, they act selectively. Gatedchannels are opened or closed by chemical or electri-cal
signals. Leakage channels are always
open. They allow water or ions to move through based on con-centration
gradients. Similar to carriers, channels can also be inhibited by some
molecules, be specific, and show saturation. The concentration gradient is also
followed in channels. As substances cross the membrane by simple diffusion, the
diffusion rate is not controlled because the lipid solubility of the membrane
is not immediately changeable. However, the facilitated diffusion rate is
controllable, because membrane permeability may be altered by regu-lating the number or activity of individual
channels (or carriers) . Membranes may be freely
permeable, selectively
permeable, or impermeable. Plasmamembranes have selective permeability because of the size,
molecular shape, electrical charge, or lipid solubility of materials as well as
other factors. Perme-ability differs because of the lipids and proteins that
are present in the plasma membrane and how they are arranged.
Osmosis
Osmosis is a special type of diffusion that occurs whenwater
molecules diffuse from an area of higher water concentration to an area of
lower water concentration. This requires a selectively permeable membrane such
as a cell membrane. Solutions containing higher con-centrations of solutes have
lower concentrations of water and vice versa. The ability of osmosis to create
enough pressure to raise a volume of water is called osmotic pressure. Water always diffuses towardsolutions
of greater osmotic pressure. Via osmosis, water equilibrates throughout the
body, so the con-centration of water and solutes in both intracellular and
extracellular fluids is nearly the same. Surpris-ingly, although highly polar,
water passes via osmosis through the lipid bilayer. This may occur because of
random movements of membrane lipids, which open small gaps in the membrane that
allow water to move through. Osmosis is very important in the determina-tion of
water distribution in the cells, blood, and other fluid-containing body
compartments. It basically con-tinues until osmotic and hydrostatic pressures
acting upon the membrane are equal.
Aquaporins are transmembrane proteins thatconstruct water- specific
channels that allow water to move freely and reversibly and water molecules to
be diffused in a single file manner. Although believed to exist in all cell
types, they are most prevalent in red blood cells and cells involved in water
balance (kid-ney tubule cells, etc.). Whenever water concentration differs on
opposite sides of a membrane, osmosis occurs. If the solute concentration
differs on either side of a membrane, water concentration also differs. When solute concentration increases, water
concentra-tion decreases.
The number (not the type) of
solute particles determines the extent to which solutes decrease water
concentration. This is because one water molecule is (basically) displaced by
one molecule or one ion of solute. Osmolarity is defined as the total concentra-tion of all solute particles in a
solution. Net diffusion of both solute and water occurs, moving down their
con-centration gradients, when the same volumes of aque-ous solutions of
different osmolarity are separated by a membrane that is permeable to all molecules in the system. When the
water and solute concentration on both sides of the membrane is the same, equilibrium is reached. Osmolarity is
based only on a solution’s total solute concentration. It is expressed as osmoles per liter (osmol/L). One osmol
is equal to 1 mole of nonioniz-ing molecules.
If a membrane is impermeable to solute parti-cles, water
diffuses quickly from the left to the right compartment. This continues until
the concentration is the same on both sides of the membrane. In this example, equilibrium
results only from the movement of water because the solutes are prevented from
mov-ing. The movement of water causes dramatic changes in the volumes of both
compartments. This is similar to osmosis
across plasma membranes of living cells. In a living plant cell, different from
the previous example, the rigid cell wall outside the plasma membrane will
eventually reach a point where water that is diffusing in will cause its hydrostatic pressure to equal
its osmotic pressure. There will then
be no further netwater entry. In general, the higher the amount of non-penetrating (nondiffusible) solutes
in a cell, the higherthe osmotic pressure. Also, this means that a greater
hydrostatic pressure must occur to resist additional net water entry. The
hydrostatic pressure pushes water out, whereas the osmotic pressure pulls water
in.
In living animal cells, these
major hydrostatic ver-sus osmotic changes do not occur because cell walls are
not as rigid. When an osmotic imbalance causes an animal cell to swell or
shrink, one of two things occurs: either the solute concentration will be the
same on both sides of the plasma membrane or the membrane will stretch until it
breaks.
Tonicity refers to a solution’s ability to changethe shape or tone of
cells by altering their internal water volume. This is not the same as
osmolarity, because tonicity is based on how a solution affects cell volume.
This is based on two factors: the solute concentration and the solute
permeability of the plasma membrane.
Any solution with the same
osmotic pressure as body fluids is called isotonic. The concentrations of nonpenetrating solutes are the same as those
found in the cells (5% glucose or 0.9% saline). When a cell is exposed to an
isotonic solution, it retains its nor-mal shape with no net gain or loss of
water. The body’s extracellular fluids and most intravenous solutions are
isotonic.
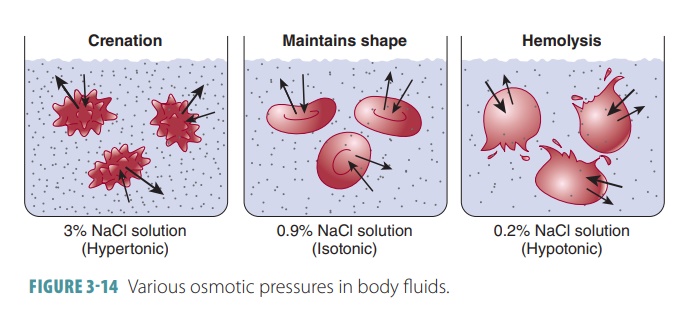
Any solution with a higher
osmotic pressure than body fluids is called hypertonic. This type of solution has a higher concentration of nonpenetrating
solutes than in the cells. Cells that receive hypertonic solutions lose water
and crenate (shrink). A strong saline solu-tion is an example of a hypertonic
solution. Likewise, any solution with a lower osmotic pressure than body fluids
is called hypotonic (FIGURE 3- 14). A hypotonic solution is more dilute with a lower concentration of
nonpenetrating solutes than cells. A cell receiving ahypotonic solution swells
quickly. The most extreme example of a hypotonic solution is distilled water,
which contains zero solutes. It causes cells to eventu-ally lyse (burst).
Hypertonic solutions are commonly
given intra-venously to patients who are swollen (edematous) because water is
retained in their tissues. These solu-tions cause the excess water to be drawn
out of the extracellular space. It is then moved into the blood to be
eliminated by the kidneys. Hypotonic solutions rehydrate tissues that have
become dehydrated. When dehydration is mild, the patient is usually given
hypo-tonic fluids such as apple juice or a “sports drink,” and rehydration
usually results.
Filtration
Filtration is a passive cell mechanism that
forces mole-cules through membranes. It is further defined as any mechanical,
physical or biological operation that sep-arates solids from fluids. The fluid
that passes through is called the filtrate.
1. Differentiate
between osmosis and diffusion.
2. Compare
simple diffusion with facilitated diffusion.
3. What is
tonicity?
4. Compare
hypertonic and hypotonic solutions.
Related Topics