Organic Substances
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Chemical Basics of Life
1. Explain the most common type of lipids and list the components their molecules contain. 2. Distinguish between saturated and unsaturated fats. 3. Define the terms phospholipid and steroid. 4. What are the roles of prostaglandins?
Organic
Substances
Organic substances include
carbohydrates, lipids, proteins, and nucleic acids. Many organic molecules are
made up of long chains of carbon atoms linked by covalent bonds. The carbon
atoms usually form addi-tional covalent bonds with hydrogen or oxygen atoms and
less commonly with nitrogen, phosphorus, sulfur, or other elements.
Carbohydrates
Carbohydrates
provide much of the energy requiredby
the body’s cells and help to build cell structures. Carbohydrate molecules
consist of carbon, hydro-gen, and oxygen molecules. The carbon atoms they
contain join in chains that vary with the type of car-bohydrate. The hydrogen
and oxygen atoms usually occur in a 2:1 ratio, which is the same as in water.
In most cases, the overall carbon to hydrogen to oxygen ratio is 1:2:1.
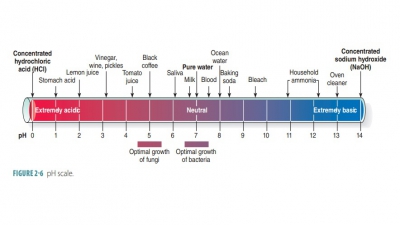
Carbohydrates with shorter chains are called sugars. Carbohydrates also include starches. Collectively, carbohydrates represent 1% to 2% of cell
mass in the body. The term carbohydrateactu-ally
means “hydrated carbon.” Usually, the larger the carbohydrate molecule, the
less soluble it is in water. Carbohydrate molecules are the body’s most readily
available source of energy.
Monosaccharides
Simple sugars have 6 carbon
atoms, 12 hydrogen atoms, and 6 oxygen atoms (C6H12O6).
They are also known as monosaccharides. Simple sugars include glucose, fructose, galactose, ribose, and deoxyribose.
Ribose and deoxyribose differ from the others in that they each contain five
atoms of carbon. The most important metabolic fuel molecule in the body is
glu-cose. Monosaccharides are single chain or single ring structures. They may
contain between three and seven carbon atoms. Monosaccharides are generally
named based on the number of carbon atoms they contain. In the human body, the
most important ones are the pentose (five-carbon) and hexose (six-carbon)
sugars (FIGURE 2-7).
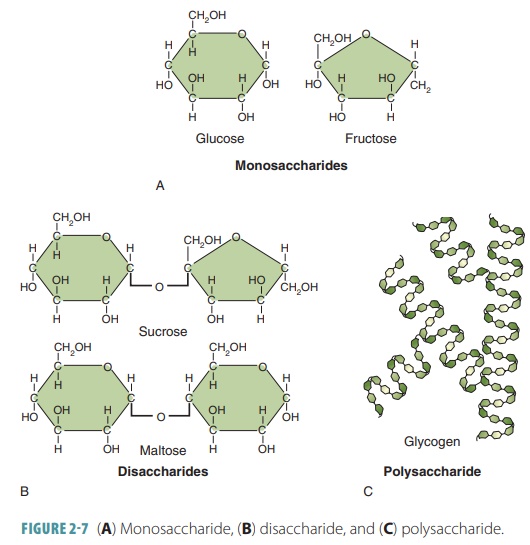
Disaccharides
Complex carbohydrates include
sucrose (table sugar) and lactose (milk sugar). Some of these carbohydrates are
double sugars or disaccharides and are formed when two monosaccharides are joined by dehydra-tion synthesis. A water molecule
is lost as the bondis made. Another important disaccharide is malt-ose(malt sugar). Disaccharides
cannot pass throughcell membranes because of their size, so instead are
digested to simple sugar units for absorption from the digestive tract. They
decompose via hydrolysis, which is
basically the reverse process of dehydration synthe-sis. A water molecule is
added, which breaks the bond and releases the simple sugar units.
Polysaccharides
Other types of complex
carbohydrates contain many simple joined sugar units such as plant starch, and
are known as polysaccharides. They are polymers of simple sugars, linked together via dehydration
synthesis, and function as storage products because they are large and fairly
insoluble. They are less sweet than the simple and double sugars. Humans and
other animals synthesize a polysaccharide called glycogen.
In all animal tissues, glycogen is the storage carbo-hydrate.
It is mostly stored in the skeletal muscle and liver, and is highly branched
(like starch) and made up of large molecules. When the blood sugar level drops
quickly, the liver cells break down glycogen releas-ing its glucose units into
the blood. Because of many branch ends that can release glucose at the same
time, body cells can have almost immediate stores of glu-cose to use as fuel.
Only glycogen and starch are of
major impor-tance in the human body. They are glucose polymers with different
forms of branching. Starch is the storage carbohydrate that
is formed by plants, with high and variable amounts of glucose units. Starches
include potatoes and grain products. Starches must be digested for absorption.
Humans cannot digest cellulose, which is another polysaccharide found in plants, but it functions as bulk, a form of fiber, which aids in
peristalsis of feces.
Carbohydrates are primarily used
by the body for ever-ready, easy- to-use cellular fuel. Glucose is the primary
form of fuel used by most cells, which in general can only use a few types of
other simple sugars. Remember that glucose is broken down and oxidized inside
cells, during which time electrons are transferred. This releases the bond
energy that is stored in the glucose, and ATP can be synthesized. When ATP is
sufficiently present, carbohydrates from the diet can be converted to glycogen
(or fat) and stored in the body. For structural needs, only tiny amounts of
carbohydrates are used. There are some sugars in human genes, whereas others
are attached to external cell surfaces and used to guide interactions between cells.
Lipids
Lipids are insoluble in water, but may dissolve inother lipids,
oils, ether, chloroform, or alcohol. Lipids include a variety of compounds such
as triglycerides, phospholipids, and steroids with vital cell functions. Fats
are the most common type of lipids. They provide roughly twice the energy of
carbohydrates. Lipids help to maintain body temperature. Like carbohy-drates,
fat molecules also contain carbon, hydrogen, and oxygen but have far fewer
oxygen atoms than do carbohydrates. Some complex lipids also contain
phosphorus. Lipoproteins are complexes or com-pounds that contain lipids and
proteins. Nearly all lipids in the plasma are present as lipoproteins. There
are five types of lipoproteins:
■■High-density lipoproteins (HDL): Good cholesterol
■■Low-density lipoproteins (LDL): Bad cholesterol
■■Very-high-density lipoproteins (VHDL)
■■Very-low-density lipoproteins (VLDL)
■■Intermediate-density lipoproteins (IDL)
Triglycerides
Fatty acids and glycerol are the
building blocks of fat molecules. A single fat molecule consists of one
glycerol molecule bonded to three fatty acid molecules. These fat molecules
are known as triglycerides, also called neutral fats, a
subcate-gory of lipids that includes fats (when solid) and oils (when liquid).
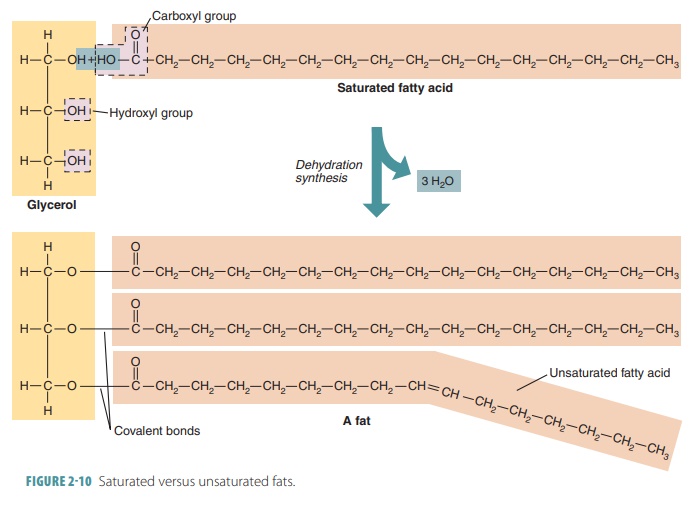
These molecules are formed by the condensation of one molecule of glycerol,
which is a three -carbon sugar alcohol (a modified simple sugar) . A
triglyceride contains three fatty acid mol-ecules and glycerol. Triglycerides contain different saturated and
unsaturated fatty acid combinations. Those with the most saturated fatty acids
are called saturated
fats and those with the
most unsaturatedfatty acids are called unsaturated
fats. In general, the
ratio of fatty acids to glycerol in a triglyceride is 3:1. Via dehydration
synthesis, fat synthesis involves the attachment of three fatty acid chains to
just one glycerol molecule. An E-shaped molecule is devel-oped. The fatty acid
chains vary, but the glycerol is always the same in all triglycerides.
Fatty acids are linear chains of
carbon and hydro-gen atoms known as hydrocarbon
chains, with an organic acid group located at one end. They consist of a
long hydrocarbon tail and a smaller
area consisting of a carboxyl group known as the head (FIGURE 2-8). Triglycerides may be made up of hundreds of atoms. Fats and oils,
after being consumed, must be broken down to their simpler building blocks
before they can be absorbed. Nonpolar molecules are made from their hydrocarbon
chains. Oils (fats) and water cannot mix because polar and nonpolar molecular
molecules cannot interact. Triglycerides provide the body’s best type of stored
energy. Upon oxidizing, they release large amounts of energy. Deeper body
tissues are protected from heat loss and mechanical trauma by triglycerides,
which are mostly found beneath the skin. Women have a thicker subcutaneous
fatty layer than men, which helps to insulate them from colder temperatures.
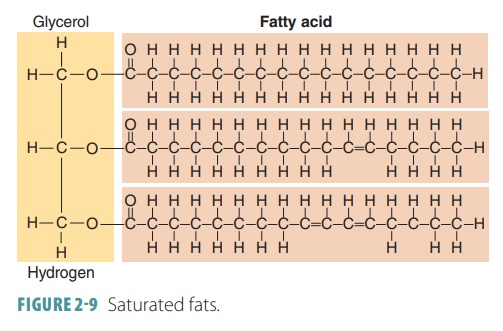
Saturated fat is defined as containing
carbonatoms that are bound to as many hydrogen atoms as possible becoming
saturated with them. The degree of saturation determines how solid the molecule
is at various temperatures. Saturated fats have fatty acid changes with single
covalent bonds between carbon atoms (FIGURE
2-9). These straight fatty acid
chains have saturated fat molecules packed closely together at room temperature
making them solid. Longer fattyacid chains and fatty acids with more saturation
are commonly found in animal fats and butterfat, which are solid at room
temperature.
Fatty acid molecules with one
double bond between carbon atoms are called unsaturated.
Double bonds cause fatty acid chains to form “kinks,” mean-ing they cannot be
packed closely enough to solid-ify. Therefore, triglycerides with either short
fatty acid chains or unsaturated fatty acids are oils. They are liquid at room
temperature, a typical factor of plant lipids. Examples include oils from corn,
olives,peanuts, safflowers, and soybeans. Unsaturated fats (especially olive
oil) are healthier. Fatty acid molecules with many double-bonded carbon atoms
are called polyunsaturated. FIGURE 2-10compares
the differencesbetween saturated and unsaturated fats.
Many types of margarines and
baked products contain trans fats, which are oils solidified by adding hydrogen atoms at the sites of
carbon double bonds. Trans fats are now known to increase risks for heart
disease even more significantly than solid animal fats. Oppositely, the omega-3 fatty acids from
coldwater fish are known to decrease the risk of heart disease and certain
inflammatory diseases.
Phospholipids
Similar to a fat molecule, a phospholipid consists
of a glycerol portion with fatty acid chains. They are structurally related to
glycolipids and are actu-ally modified triglycerides. Human cells can
synthe-size both types of lipids, primarily from fatty acids. A phospholipid
includes a phosphate group that is soluble in water and two molecules of fatty
acids. They are an important part of cell structures. The distinc-tive chemical
properties of phospholipids come from the phosphorus-containing group. The
tails of these molecules (the hydrocarbon portion) are nonpolar; they react
only with nonpolar molecules. The heads of these molecules (the
phosphorus-containing part) are polar, attracting other polar or charged
particles (including ions or water). The unique phospholipids can be used as
the primary material for the building of cell membranes.
Steroids
Steroid molecules are large, basically flat lipid mole-cules that
share a distinctive carbon framework in com-parison with fats or oils. Steroids
have four connected rings of carbon atoms. All steroid molecules have the same
basic structure: three six-carbon rings joined to one five-carbon ring. They
include cholesterol, estro-gen, progesterone, testosterone, cortisol, and
estradiol (FIGURE 2-11). Steroids are also fat soluble and have little to no
oxygen. Steroid hormones are vital for homeosta-sis. The sex hormones include
the sex steroids, which are essential for reproduction. If no corticosteroids were produced by the adrenal glands, it would be fatal.
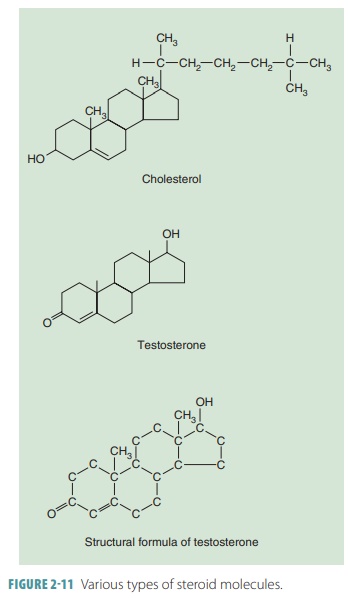
Cholesterol is the most important steroid and
isingested in animal foods such as cheese, eggs, and meat. The liver also
produces certain amounts of cho-lesterol. Although essential for human life,
excessive cholesterol participates in atherosclerosis and related disease. In
the cell membranes, cholesterol is the raw material that helps to synthesize
vitamin D, bile salts, and steroid hormones.
Eicosanoids
Eicosanoids are lipids that are mostly derived from arachidonic acid, a 20-carbon fatty acid existing inall cell membranes, the most important of which are the prostaglandins and related acids. Prostaglandins are important for blood clotting, inflammation, labor contractions, regulation of blood pressure, and many other body processes. Prostaglandin synthesis and inflammatory effects are blocked by medications such as the cyclooxygenase inhibitors and nonsteroidal anti-inflammatory drugs.
1. Explain
the most common type of lipids and list the components their molecules contain.
2. Distinguish
between saturated and unsaturated fats.
3. Define
the terms phospholipid and steroid.
4. What
are the roles of prostaglandins?
Proteins
Proteins are the most abundant organic componentsof the human body
and in many ways the most import-ant. They make up between 10% and 30% of cell
mass and are the basic structural materials of the body. Pro-teins are vital
for many body functions. On cell sur-faces, some proteins combine with
carbohydrates to become glycoproteins.
They allow cells to respond to certain molecules that bind to them. Proteins
include biologic catalysts (enzymes), contractile proteins of muscles, and the
hemoglobin of the blood.
There are more than 200,000 types
of proteins in the human body, the full set known as the proteome.
Antibodies are proteins that detect and destroy foreign substances. All
proteins contain carbon, hydrogen, oxygen, and nitrogen atoms, with small
quantities of sulfur also present. Proteins always con-tain nitrogen atoms.
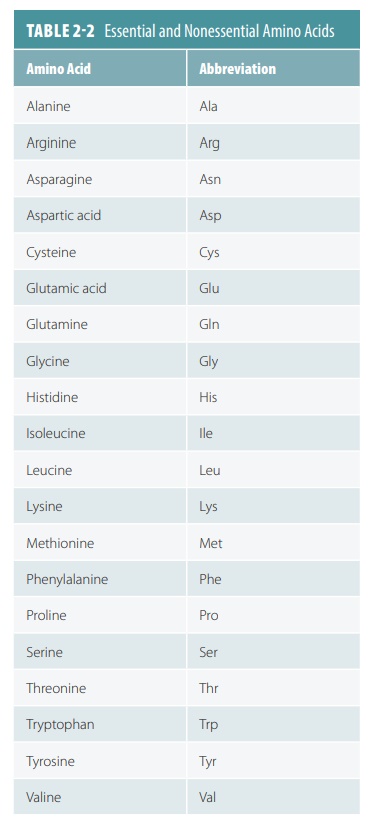
Twenty common amino acids, both essential and nonessential, make up the proteins that exist in
humans and most other living organ-isms (TABLE
2-2).
Amino acids are the building
blocks of proteins, with two primary groups: amines and organic acids.
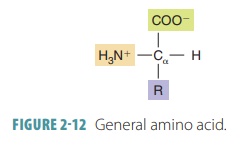
Amino acids act as either bases (protonacceptors) or acids (proton donors). All
amino acids are exactly the same except for one group of atoms, known as the
amino acid’sR group. Differences in
the R group determine the chemical uniqueness of each amino acid (FIGURE 2-12).
Nucleic Acids
Nucleic acids
are large organic molecules
(macro-molecules) that carry genetic information or form structures within
cells. They are composed of car-bon, hydrogen, oxygen, nitrogen, and
phosphorus. Nucleic acids are actually the largest molecules in the body.
Nucleic acids store and process information at the molecular level inside the
cells. The two classes of nucleic acids are deoxyribonucleic
acid (DNA) and ribonucleic acid (RNA). Nucleic acids are found in allliving things, cells, and viruses.
Individual strands of DNA and RNA have a similar structure (FIGURE 2-14).
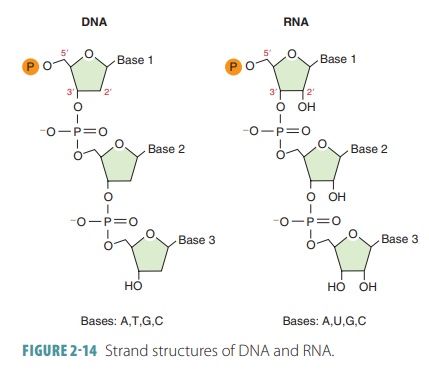
Nucleotides are the structural units of
nucleicacids. These complex units consist of a nitrogen- containing base, a
pentose sugar, and a phosphate group. The nucleotide structure is based on five
major types of nitrogen-containing bases:
■■ Adenine (A): A large,
two-ring base (purine)
■■ Guanine (G): Also a purine
■■ Cytosine(C): A smaller, single-ring base (pyrimidine)
■■ Thymine (T): Also a
pyrimidine
■■ Uracil (U): Also a pyrimidine
Enzymes
Enzymes are globular proteins that promote chem-ical reactions by lowering the activation energy requirements. Activation energy is the energy that must be overcome for a chemical reaction to occur. Therefore, they make chemical reactions possible and catalyze the reactions that sustain life. This means that enzymes are catalysts. Enzyme molecules are manufactured by cells to promote specific reac-tions. Enzymes are among the most important of all the body’s proteins. Nearly everything that occurs in the human body relies on a specific enzyme. In the body, enzymes assist in the digestion of food, drug metabolism, protein formation, and many other types of reactions. Enzymes make metabolic reac-tions possible inside cells by controlling tempera-ture conditions that otherwise would be too mild for them to occur.