Adenosine Triphosphate
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Chemical Basics of Life
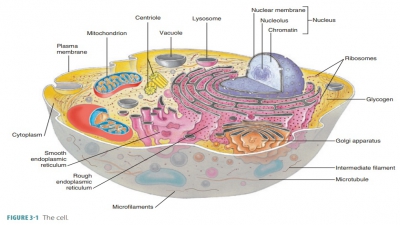
Although glucose is the primary cellular fuel, it does not directly power cellular work. When glucose is catabolized, the released energy is paired with the synthesis of adenosine triphosphate (ATP).
Adenosine
Triphosphate
Although glucose is the primary
cellular fuel, it does not directly power cellular work. When glucose is
catabolized, the released energy is paired with the synthesis of adenosine triphosphate (ATP). Some of the released energy is stored by ATP bonds in small “packets.”
Therefore, ATP is the main molecule in cells that transfers energy. It provides
a type of energy that all body cells can use immediately.
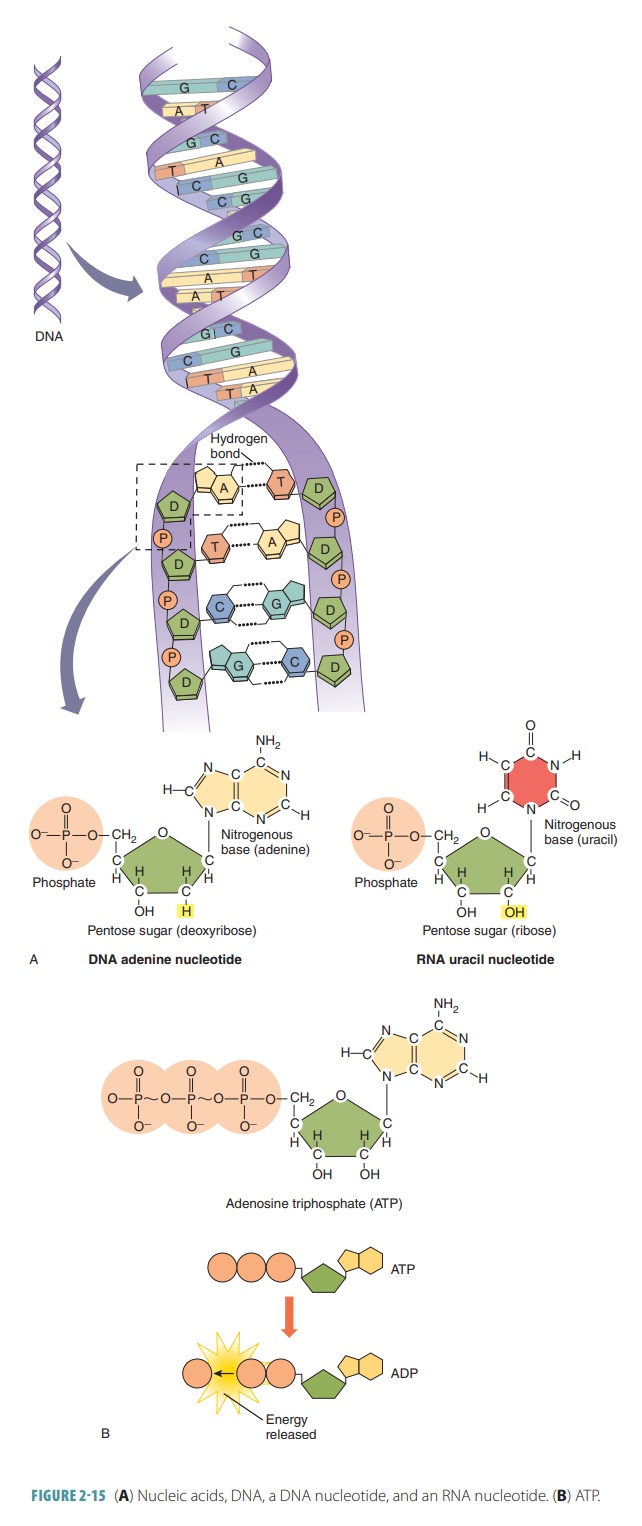
The structure of ATP is an
adenine-containing RNA nucleotide, with two additional phosphate groups added
(FIGURE 2-15B). The triphosphate “tail” of ATP is ready to chemically release
enormous amounts of energy. ATP is a highly unstable molecule that stores
energy, because its three phosphate groups are negatively charged and closely
packed. They repel each other, and when the terminal high-energy phos-phate bonds
are hydrolyzed, the molecule becomes more stable.
The ATP bond energy is taken by the cells during coupled reactions. The cells use enzymes to transfer terminal phosphate groups from ATP to various com-pounds. The newly phosphorylated molecules tempo-rarilyhave higher energy and can perform various types of cellular work. During this work, the mole-cules lose the phosphate group. The amount of energy needed for most biochemical reactions is closely related to the amount of energy released and trans-ferred during the hydrolysis of ATP. Therefore, thecells are not damaged by an excessive energy release, and so very little energy is wasted.
When the terminal phosphate bond
of ATP is cleaved, a molecule is given off that has two phosphate groups: adenosine diphosphate (ADP) and an
inorganic phosphate group. There is a transfer of energy. ADP accumulates as
ATP is hydrolyzed for cellular energy needs. When the terminal phosphate bond
of ADP is cleaved, a similar amount of energy is liberated. This produces adenosine monophosphate.
As glucose and other
fuel-providing molecules are oxidized, their bond energy is released and ATP
sup-plies in the cells are replenished. The same amount of energy is needed to
be acquired as is released when the terminal phosphates of ATP are cleaved.
This energy must be used to reverse the reaction, reattaching phos-phates and
reforming energy-transferring phosphate bonds. Molecules cannot be made or
degraded with-out ATP. Also, without ATP, cells cannot transport substances
across boundaries, muscles cannot shorten to pull on other body structures, and
the process of life will cease.
Certain compounds may be lethal
because they interfere with the mitochondrial production of ATP. An example is
cyanide, which when inhaled or absorbed has extreme effects on the brain and
heart. When the cells do not have sufficient ATP, they die because they lack
the energy needed for anabolic chemical reactions, active transport, and other
cell processes.
Related Topics