Background
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Risk Management - a European Regulatory View
The test of the effectiveness of regulatory systems is their performance in responding to emerging drug safety hazards to identify, evaluate, manage and communicate risk in the context of benefit.
BACKGROUND
The
test of the effectiveness of regulatory systems is their performance in
responding to emerging drug safety hazards to identify, evaluate, manage and
communicate risk in the context of benefit. Recent significant drug safety
issues handled in the European regulatory framework and the evidence which
trig-gered them are shown in Table 44.1.
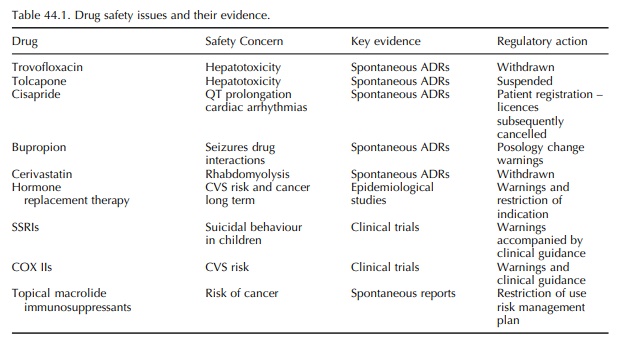
The
withdrawal of cerivastatin in 2001 following spontaneous reports of cases of
serious and fatal rhab-domyolysis represented a regulatory milestone. The
extent of use of cerivastatin in Europe meant that wide public debate ensued.
This debate was reignited on an international scale in September 2004, when
Merck withdrew rofecoxib, a selective cyclo-oxygenase 2 inhibitor widely used
in the treatment of arthritic pain, because of clinical trial evidence of an
increased risk of heart attack and stroke.
While
high-profile drug withdrawals have been the focus of detailed public scrutiny,
evidence has contin-ued to gather of the general burden of adverse drug
reactions in public health terms. Research conducted in the United States by
Lazarou concluded that adverse drug reactions in 1994 were between 4th and 6th
leading cause of death (Lazarou et al.,
1998). A recent prospective analysis in the United Kingdom by Pirmohamed et al.
gave a similar estimate; about 6.5% of hospital admissions were related to an
adverse drug reaction (ADR) with a 0.15% incidence of fatal ADRs (Pirmohamed et al., 2004).
Related Topics
