Batch Dryers
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Drying
Batch Dryers: Hot Air Ovens, Vacuum Tray Dryers, Tumbling Dryers, Fluidized Bed Dryers, Agitated Batch Dryers, Freeze-Drying
BATCH DRYERS
Hot Air Ovens
Ovens
operating by passing hot air over the surface of a wet solid that is spread
over trays arranged in racks provide the simplest and cheapest dryer. On small
installations, the air is passed over electrically heated elements and once
through the oven. Larger units may employ steam-heated, finned tubes, and
thermal efficiency is improved by recirculating the air. This is controlled by
manually set dampers, and a common operating position gives 90% recircula-tion
and 10% bleed-off. The heater bank is placed so that the solids do not receive
radiant heat and incoming air may be filtered. A typical hot air oven is
illustrated schematically in cross section in Figure 7.7A.
The
temperature-humidity sequence of the circulating drying air is pre-sented in
Figure 7.7B. The incoming air, at a temperature and humidity given by point A,
is heated at constant humidity to point B and passed over the wet solid. The
humidity rises and the temperature falls as the adiabatic cooling line is
followed until the air leaves the tray in condition C. It is then recirculated
to the heater, and in Figure 7.7B, two further cycles are shown.
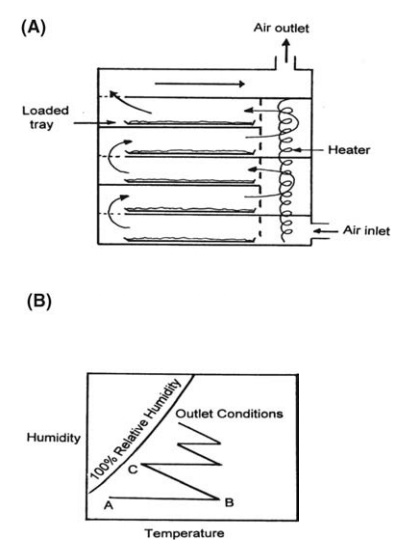
FIGURE 7.7 (A) A tray dryer. (B) Temperature-humidity sequence of drying air.
We
have assumed that all heat is drawn from the air and transmitted across the
stationary air layer in contact with the drying surface, as described earlier.
Surface temperatures are, in fact, modified by heat absorbed and con-ducted
from unwetted surfaces, such as the underside of the tray, and by radiation.
The
chief advantage of the hot air oven, apart from its low initial cost, is its
versatility. With the exception of dusty solids, materials of almost any other
physical form may be dried. Thermostatically controlled air temperatures of
between 408C and 1208C permit heat-sensitive materials to
be dried. For small batches, a hot air oven is, therefore, often the plant of
choice. However, the following inherent limitations have led to the development
of other small dryers:
1. A large floor space is required for the oven and
tray-loading facilities.
2. Labor costs for loading and unloading the oven are high.
3. Long drying times, usually of the order of 24 hours, are
necessary.
4. Solvents can be recovered from the air only with
difficulty.
5. Unless carefully designed, nonuniform distribution of air
over the trays gives variation in temperature and drying times within the oven.
Variations of ±78C in temperature
have been found from location to location during the drying of tablet granules.
Poor air circulation may permit local saturation and the cessation of drying.
If
the material is of suitable granular form, drying times may be reduced to an
hour or less by passing the air downward through the material laid on mesh
trays. The oven in this form is called a batch through-circulation dryer.
Vacuum Tray Dryers
Vacuum
tray dryers, as shown in Figure 7.8A, differing only in size from the familiar
laboratory vacuum ovens, offer an alternative method for drying small
quantities of material. When scaled up, construction becomes massive to
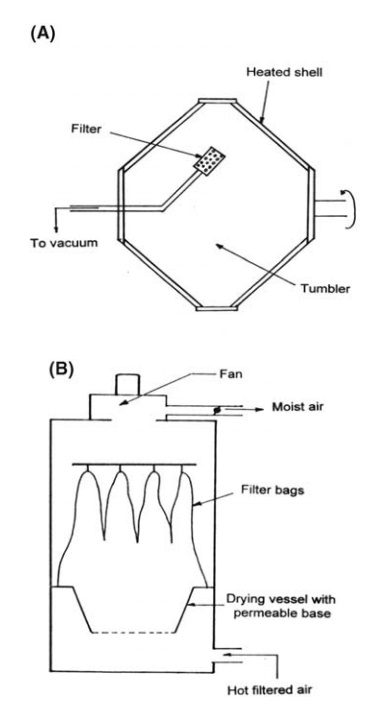
FIGURE 7.8 (A) Rotary vacuum dryer and (B) fluidized bed dryer.
withstand
the applied vacuum, and cost is further increased by the associated vacuum
equipment. Vacuum tray dryers are, therefore, only used when a def-inite
advantage over the hot air oven is secured, such as low-temperature drying of
thermolabile materials or the recovery of solvents from the bed. The exclusion
of oxygen may also be advantageous or necessary in some operations.
Heat
is usually supplied by passing steam or hot water through hollow shelves.
Drying temperatures can be carefully controlled, and for the major part of the
drying cycle, the material remains at the boiling point of the wetting liquid
under the operating vacuum. Radiation from the shelf above may cause a significant
increase in temperature at the surface of the material if high drying
temperatures are used. Drying times are long and usually of the order of 12 to
48 hours.
Tumbling Dryers
The
limitations of ovens, particularly with respect to the long drying times, have,
where possible, promoted the design and application of other batch dryers. The
simplest of these is the tumble drier for which the most common shape is the
double cone shown in Figure 7.8A. Operating under vacuum, this provides
controlled low-temperature drying, the possibility of solvent recovery, and
increased rates of drying. Heat is supplied to the tumbling charge by contact
with the heated shell and by heat transfer through the vapor. Optimum
con-ditions are established experimentally by varying the vacuum, the
temperature, and, if the material passes through a sticky stage, the speed of
rotation. With correct operation, a uniform powder should be obtained as
distinct from the cakes produced when static beds are dried. Some materials,
such as waxy solids, cannot be dried by this method because the tumbling action
causes the material to aggregate into balls.
A
normal charge would be about 60% of the total volume, and for dryers 0.7 to 2 m
in diameter, drying times of 2 to 12 hours may be expected. In studying the
application of tumbler dryers to drying tablet granules, it was found that
periods of 2 to 4 hours replaced times of 18 to 24 hours obtained with hot air
ovens. The mixing and granulating capacity of the tumbling action has suggested
that these operations could precede drying in the same apparatus.
Fluidized Bed Dryers
The
term “fluidization” is applied to processes in which a loose, porous bed of
solids is converted to a fluid system having the properties of surface
leveling, flow, and pressure-depth relationships by passing the fluid up
through the bed.
Fluidized
bed techniques, employing air as the fluidizing medium, have been successfully
applied to drying when the solid is of suitable physical form. The high
interfacial contact between drying air and solids gives drying rates 10 to 20
times greater than that obtained during tray drying. A drying curve for this
method is shown in Figure 7.9.
The
dryer, illustrated in Figure 7.8B, consists of a basket of either plastic or
stainless steel with a perforated bottom, which is mounted in the body of the
drier and into which the material to be dried is placed. Heated air may be
either blown or sucked through the bed. The air leaving the basket passes
through an air filter and may be recirculated. Particle properties, such as
shape and size distribution, affect fluidization, and a unit must have a
variable air flow adjusted
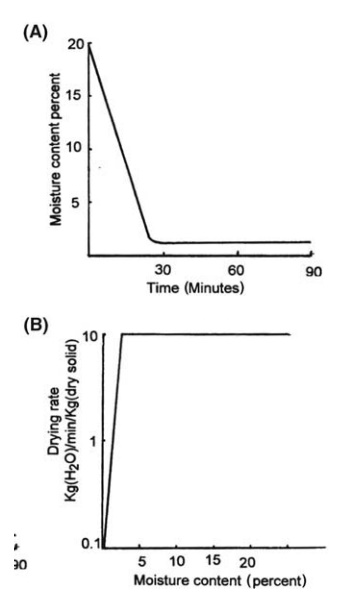
FIGURE 7.9 Drying curves.
so
that the material is fluidized but is not carried into the filters. For this
reason, the material must have a fairly close size range or else elutriation of
fine particles into the filters will take place.
Fluidized
bed dryers are particularly suitable for granulated materials and are being
increasingly used for tablet granulations provided product changeover is not
too frequent. It may be advantageous to preform other materials, such as a
dewatered filter cake, into granules solely to employ fluidized bed drying. If
fluidizing conditions are ideal, the granulation will not require further
grinding. Tray dryers, on the other hand, produce a caked product that may
require mild comminution. Variation in temperature, which may be quite marked
in tray dryers, is virtually eliminated in fluidized bed dryers by the intense
mixing action. The floor space for a given capacity is smaller compared with a
tray dryer. Machines vary in size, handling up to 250 kg. Drying times,
maximum, minimum, and optimum air velocities, air temperature, and the tendency
to cake and channel are established experimentally as these cannot be predicted
accu-rately at present.
Considerable
erosion and the production of large amounts of fines might be expected from the
intense turbulent movement. Experience shows that the opposite is true. The
particles are to some extent “padded” by the surrounding fluid so that either
the amount of contact between particles is low or the impact energy is small.
Agitated Batch Dryers
Agitated
batch dryers consist of a jacketed cylindrical vessel with agitator blades designed
to scrape the bottom and the walls. The body may be run at atmo-spheric
pressure or under vacuum. Pasty materials, which could not be handled in
tumbling or fluidized bed dryers, may be successfully dried at rates higher
than that can be achieved in an oven.
Freeze-Drying
Freeze-drying
is an extreme form of vacuum drying in which the solid is frozen and drying
takes place by subliming the solid phase (Dushman and Lafferty, 1962; Jennings,
1988; Nail, 1980; Pikal et al., 1984). Low temperatures and pressures are used.
Establishing and maintaining these conditions, together with the low drying
rates obtained, create a most expensive method of drying, which is only used on
a large scale when other methods are inadequate.
There
are two principal fields in which freeze-drying is extensively used. It is used
when high rates of decomposition occur during normal drying. The second field
concerns substances that can be dried at higher temperatures but are thereby
changed in some way. Fruit juices, for example, are reputed to lose subtle
elements of flavor and odor, and proteinaceous materials are partly denatured
by the concentration and higher temperatures associated with con-ventional
drying. Drying of blood plasma and some antibiotics are important large-scale
applications of freeze-drying. On a smaller scale, it is extensively used for
the dehydration of bacteria, vaccines, blood fractions, and tissues.
Freeze-drying
is theoretically a simple technique. Pure ice exhibits an equilibrium vapor
pressure of 4.6 mmHg at 08C
and 0.1 mmHg at – 408C.
The vapor pressure of ice containing dissolved substances will, of course, be
lower. If, however, the pressure above the frozen solution is less than its
equilibrium vapor pressure, the ice will sublime, eventually leaving the solute
as a sponge-like residue equal in apparent volume to the original solid and,
therefore, of low bulk density. The latter is readily dissolved when water is
added, and freeze-drying has been called “lyophilic drying” or “lyophilization”
for this reason. No concentration, in the normal sense of the word, occurs, and
structural changes in, for example, protein solutions, are minimized.
In
practice, many difficulties are encountered. Under conditions of high vacuum,
water vapor must be trapped or eliminated. To maintain drying, heat must be
supplied to the frozen solid to balance the latent heat of sublimation without
melting the frozen solid. Difficulties become acute if, like blood plasma, the
product is dried in the final container under aseptic conditions.
In
the first stage of the process, the material is cooled and frozen. If the
temperature of a dilute solution of a salt is slowly reduced, leveling occurs
in the time-temperature curve just below 08C because of the liberation of the
latent heat of fusion of ice, and pure ice separates. With further cooling, the
solution becomes concentrated until the eutectic mixture is formed. This
freezes to give a plateau in the cooling curve. It is a clear indication of
complete freezing. If the concentration of the liquid eutectic mixture is
small, the material may appear to be completely frozen at higher temperatures.
Under these conditions, some drying from a liquid phase will occur, possibly
with damaging results. This can be detected by measuring the electrical
resistance of the ice that becomes infi-nitely great when the eutectic mixture
freezes. Conversely, thawing gives a marked decrease in resistance, an effect
that can be used to automatically control the state of the drying solid.
Protein solutions do not give clearly defined eutectic points and are usually
frozen to below –258C
before drying. Freezing is carried out quickly to prevent concentration of the
solution and to produce fine ice crystals. Some degree of supercooling may be
induced, followed by a very quick freeze. Freezing may or may not be carried
out in the drying chamber. If drying in final containers is necessary,
small-scale operations may employ immersion in a coolant such as liquid air or
isopentane. Larger-scale installa-tions may cool with a blast of very cold air.
Alternatively, evaporative freezing, in which the liquid is cooled to near its
freezing point and the system is rapidly evacuated, is employed. The
evaporating liquid cools and freezes rapidly. Frothing caused by the evolution
of dissolved gases may complicate this technique. For bulk drying, the liquid
is placed in shallow trays on refrigerated shelves in the drying cabinet.
A
suitable surface area to depth of solid ratio must be provided to facilitate
drying. Thin layers of frozen liquid are used in bulk drying. The surface area
of bottle-dried plasma may be increased by spinning in a vertical axis during
freezing to give a frozen shell about 2 cm thick around the inside periphery of
the bottle. Spinning also prevents frothing during evaporative freezing by
inhibiting the formation of bubbles.
In
plasma processing, freezing, and drying, handling must be carried out
aseptically. This is maintained by a filter at the neck of the bottle that
allows the passage of water vapor but prevents the ingress of bacteria. Similar
precautions are taken during the drying of antibiotics.
Effective
drying vacuum of 0.05 to 0.2 mmHg may be provided by directly pumping water
vapor and permanent gases, originally present or derived from the drying
material and from leaks, out of the system. Normal practice, how-ever, favors
interposing a refrigerated condenser between the drying surface and the pump.
This arrangement allows a smaller pump, handling mainly permanent gases, to be
used but demands a low condenser temperature, such as –50○C, to remove water
vapor at the low operating pressure. A system for bulk drying in trays is
represented diagrammatically in Figure 7.10A.
During
drying, heat must be supplied to the drying surface. When drying a material,
such as plasma, in a final container, a temperature gradient is established
across the container wall and through the ice to the drying surface by means of
a heater suitably mounted in relation to the container. The power dissipated by
the heater must be carefully controlled so that melting does not occur at the
ice-container junction, the point nearest to the heat source and at the highest
temperature. At any time, the conditions prevailing are such that the rate of
evaporation is approximately constant and temperatures and pressure adjust so
that there is a temperature and pressure gradient from the drying surface to
the condenser. As evaporation proceeds, a drying line recedes into the solid.
With the thinning of the ice layer, the temperature gradient through the ice
will be modified by the decreasing resistance to heat flow. An increase in the
rate of drying due to increase in temperature and vapor pressure of the drying
surface might, therefore, be expected. In practice, this is modified by the
layer of dried plasma that offers considerable resistance to the flow of vapor.
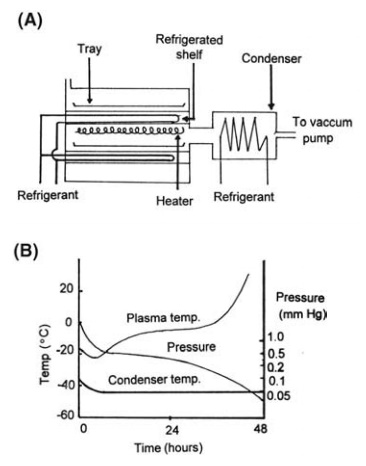
FIGURE 7.10 (A) Equipment for freeze-drying
bulk liquids in trays and (B) variations in tem-perature and pressure during
the freeze-drying cycle for blood plasma.
The
bacterial filter also causes a large, constant pressure drop. Evaporation of
pure ice without the filter and plasma layer would be 300 times faster. When
the plasma is nearly dry, its temperature is allowed to rise to about 308C to facilitate final drying. The
total drying time is about 48 hours. The temperatures and pressure in the
system during this period are shown, as a function of time, in Figure 7.10B.
If
the product is not being dried in its final container, radiant heat may be used
to provide the latent heat of sublimation. If the dried solid could be removed
continuously, high drying rates are possible. Not only is heat provided
directly to the drying surface, but there is also little danger of melting the
ice at the container wall.
Related Topics
