Biochemical Tests (or Properties)
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Identification of Microorganisms
The most vital and important and abundantly employed biochemical tests are as described below with appropriate explanations whenever required in the course of the prevalent discussion :
BIOCHEMICAL
TESTS (OR PROPERTIES)
Extensive
and meticulous in depth investigations carried out on a host of fermentative
proce-dures using different types of substrates exclusively dependent upon a
broad-spectrum of biochemical tests ultimately lead to the production
of ethanol by yeast ; acetylmethylcarbinol ; lactic acid ; acetic acid ; ethanol by E.
coli ; acetone plus CO2 ; citric acid (Krebs Cycle) ; and CO2
plus H2.
The most
vital and important and abundantly employed biochemical tests are as described below with appropriate
explanations whenever required in the course of the prevalent discussion :
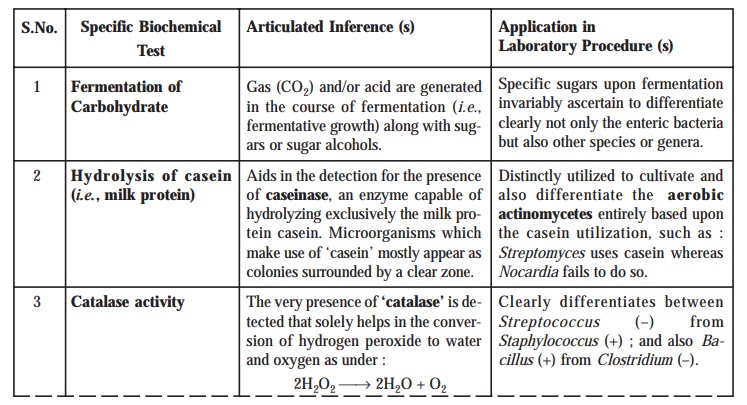
1. Carbohydrate (Sugar) Fermentation
The carbohydrate fermentation is normally
tested in a ‘sugar media’. Thus, the
generation of ‘acid’ is indicated by
an apparent change in the colouration of the ensuing medium either to pink or
red, and the resulting gaseous products produced gets duly
collected in a strategically placed Durham’s
Tube.
2. Litmus Milk
In this
particular instance there may not be any change in the medium, or acid or
alkali could be generated thereby giving rise to clotting of milk, and peptonizaiton
or saponification may take place
appreciably. The resulting ‘clot’ i.e., coagulation of the milk protein (viz., casein) could face a disrup-tion by virtue of the gas evolved (usually
termed as ‘stormy fermentation’).
3. Indole Production
In actual
practice the ‘indole production’ is
normally tested in a peptone-water
culture after an interval of 48 or 96 hours incubation at 37°C ; whereby
the generation of indole from the
amino acid tryptophan is duly
ascertained as given below :
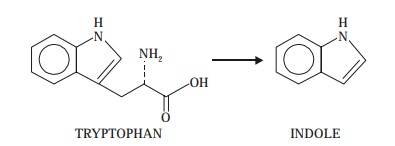
When Kovak’s Reagent*, 0.5 mL, is added
carefully and shaken gently for a while, it yields a red colouration thereby
indicating a positive reaction i.e., indole production.
4. Methyl Red Test [MR-Test]
The MR-test is frequently used to carry out
the detection for the ‘production of
acid’ in the course of fermentation of glucose,
besides maintaining pH below 4.5 in an old
culture medium [methyl red : 4.2 (red) to 6.3 yellow].
Procedure : Five droplets of methyl red
solution [0.04% (w/v)] are added into the culture in glucose-phosphate medium that had been previously incubated at
30°C for 5 days, mixed well, and read
instantly. Appearance of red colour
(acidic) gives a positive test,
whereas yellow colour repre-sents a negative test.
5. Voges-Proskauer Test [VP-Test]
The
underlying principle of the VP-Test
exclusively rests upon the production of acetyl
methyl carbinol from pyruvic
acid via an intermediate stage
in its strategic conversion to form 2,
3-butylene glycol i.e., [CH3CH-(OH)CH(OH)CH3].
However, it has been duly observed that in the presence of alkali and atmospheric oxygen (O2) the
relatively small quantum of acetyl methyl carbinol present in the medium gets oxidized to the
corresponding ‘diacetyl derivative’
that subsequently interacts with the peptone
content in the ‘culture broth’ to
produce a distinct red colouration.
Procedure : The VP-Test may be easily performed by the careful addition of 0.6 mL
of a 5% (w/
solution
of α-naphthol in ethanol and 0.2 mL solution
of 40% (w/v) KOH to 1 mL of a glucose
phosphate medium culture of the
ensuing organism previously incubated duly either at 30°C for a duration of 5 days or at 37°C for 2
days. Thus :
Positive Reaction : indicated
by the appearance of a pink colouration in
just 2-5 minutes, that ultimately
gets deepened either to magenta or crimson red in about 30 minutes
duration ;
Negative Reaction : Designated
by the appearance of a colourless solution upto 30 minutes.
Importantly,
the development of any traces of pink
colouration must be ignored
completely.
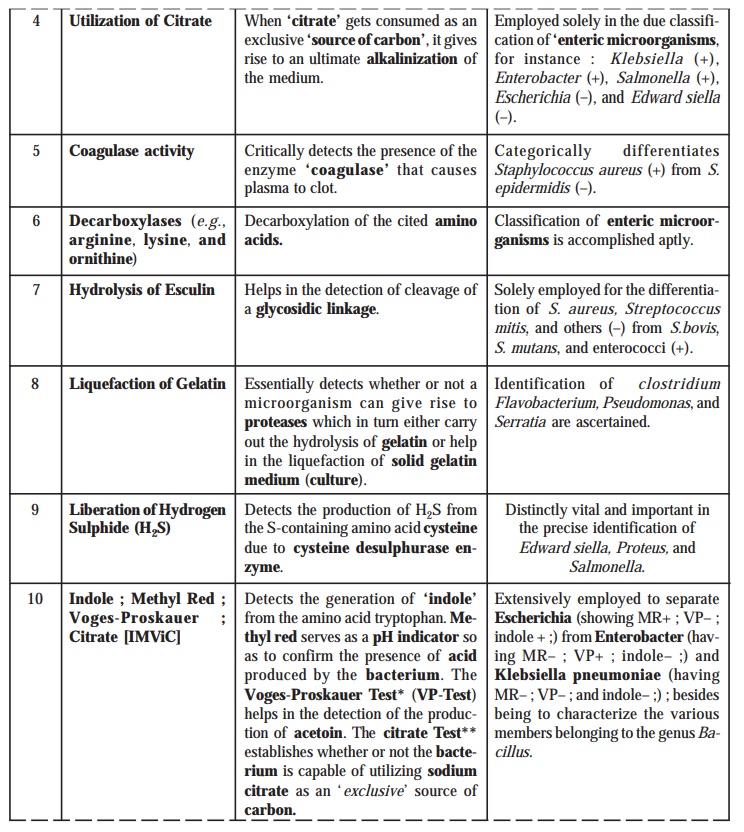
6. Citrate Utilization
In actual
practice, Koser’s citrate medium
containing ‘citric acid’ serves as
the exclusive source of carbon.
Evidently, the ability as well as the efficacy for the ‘citrate utilization’ (i.e.
, the prevailing substrate) is
adequately indicated by the production of reasonably measurable turbidity in
the medium.
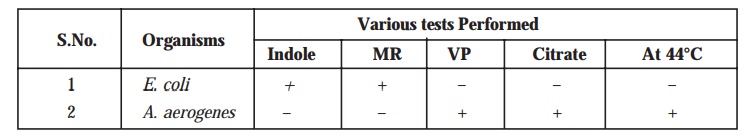
Note : The various biochemical characteristic tests
viz., indole, MR, VP, and citrate are
quite useful in the proper and prompt identification of Gram-negative
microorganisms. Hence, these tests are frequently referred to by the Sigla
‘IMVIC’ Tests.
Alternatively,
another cardinal physiological difference that may be exploited specifically
per-tains to the ensuing ‘growth
temperature’. It has been duly demonstrated that at 44°C only A. aerogenes shall display growth
particularly, whereas E. coli will
not. Therefore, the specific incubation at 44°C shall be able to make a clear
cut distinction between these two microorganisms which is invariably known as
the Eijkman (E) test. The menomic i.e., aiding the memory is IMVEC,
wherein E stands for Eijkman. Conclusively, the four cardinal tests are normally distinguished by mnemonic IMVIC or
when the Eijkman test is also included, IMVEC, and several texts predominantly
refer to the IMVIC or IMVEC characteristic features of these and other, related
organisms.
Summararily,
therefore, the apparent behaviour of the said two microorganisms may be stated as below, whereby a sort of
comparison between E. coli and A. aerogenes
has been recorded :
7. Nitrate Reduction
The ‘nitrate reduction’ test is carried out
after allowing the specific bacterium to grow for 5 days at 37° C in a culture
broth containing potassium nitrate [1% (w/v)]. The test reagent comprises of a
mixture of equal volumes of the solutions of sulphanilic acid and α-naphthylamine in
acetic acid carefully mixed just before use. Now, 0.1 mL of the test reagent is duly incorporated to the
culture medium. The results of the test may be inferred as given under :
Positive Reaction : Development
of a red colouration within a short span of a few minutes confirms a positive
reaction.
Negative Reaction : The
critical absence of the above mentioned red colouration signifies a negative reaction.
Importance : The ‘nitrate reduction’ test indicates particularly the presence of
the enzyme nitrate reductase that
helps to reduce nitrate to nitrite.
8. Ammonia Production
The ‘ammonia production’ test is usually
performed by incorporating very carefully the Nessler’s Reagent* into
a peptone-water culture grown
meticulously for 5 days at 37°C. The inferences of this test may be drawn as stated under :
Positive Test : Appearance of a Brown colour ;
Negative Test : Appearance of faint Yellow colour.
9. Urease Test
The ‘test’ is usually carried out in Christensen’s Urea-Agar medium or Christensen’s urease medium.
Procedure. The slope is inoculated profusely
and incubated at 37°C. The slope is duly examined at intervals of 4 hours and 24 hours incubation. The test must not
be taken as negative till after a duration of 4 days after incubation.
Result : The urease positive cultures give
rise to a distinct purple-pink colouration*. The exact mechanism may be explained by virtue
of the fact that urease producing microorganisms largely help in the conversion
of urea to ammonia** (gas) which is particularly responsible for the desired
colouration.
10. Production of Hydrogen Sulphide (H2S)
Importantly,
there are several S-containing amino acids e.g.,
cystine, cysteine, methionine that may decompose certain organisms to yield H2S
(gas) amongst the products of microbial degradation. In this particular
instance lead acetate [Pb(CH3CO)2]***
is duly incorporated into the culture media which eventually gets turned into
either black or brown due to the formation of PbS as given below :

Procedure : The organisms are grown in
culture tubes. In actual practice a filter-paper strip soaked in a lead acetate solution [10% (w/v) freshly prepared] is
strategically inserted between the cotton plug and the empty-space in the
culture tube.
Result : The gradual browning of the
filter paper strip rightly confirms the H2S-production.
11. Reduction of Methylene Blue
The
reduction of 1 drop of the aqueous methylene
blue reagent [1% (w/v) added into the broth culture and incubated at 37°C.
The results are as indicated below :
Strongly positive : exhibited
by complete decolourization
Weakly positive : displayed
by green colouration.
12. Production of Catalase [Tube catalase Test]
In this
specific test a loopful (either a wooden applicator stick or a nichrome wire loop) H2O2
i.e., hydrogen peroxide (3%) is
placed meticulously right upon the colonies grown on the nutrient agar medium. The catalase production is indicated by the prompt effervescence of
oxygen (O2) due to the fact that the enzyme catalase aids in the conversion of H2O2 into water and oxygen bubbles (in the form of effervescence).
Importance : It has the unique means of
differentiation between Streptococcus (catalase nega-tive)
from Staphylococcus (catalase positive).
Caution : Such ‘culture
media’ that specifically contain blood as an integral component are
definitely not suitable for the ‘tube catalase test’ because the blood itself
contains the enzyme catalase.
13. Oxidase Reaction
The
underlying principle of the ‘oxidase
reaction’ is exclusively by virtue of an enzyme known as cytochrome oxidase that particularly
catalyzes oxidation of reduced cytochrome by oxygen.
Procedure : A solution of tetramethyl p-phenylene
diamine dihydrochloride [concentration 1.0
to 1.5% (w/v)] is poured gently as well as carefully over the colonies. The
result is duly indicated by the oxidase positive
colonies turning into maroon-purple-black in a span of 10 to 30 minutes.
Kovac’s Method : Alternatively, the ‘oxidase reaction’ may also be
performed by Kovac’s method. In this
method a strip of filter paper is adequately moistened with a few drops of 1%
(w/v) solution of tetramethyl p-phenylene diamine dihydrochloride. By
the help of a sterilized wooden appli-cator the actual growth from an agar
medium is carefully smeared onto the exposed surface of the said strip of
filter paper. Thus, a positive test
is invariably indicated by the distinct development of a purple colouration
almost promptly (within 10 seconds).
Importance : The obvious importance of the ‘oxidase reaction’ is judiciously employed to obtain a clear cut differentiation/separation of the enterics from the pseudomonads.
Example : Pseudomonads
aeruginosa :
Positive Test.
Escherichia coli : Negative Test.
14. Egg-Yolk Reaction
It has
been duly demonstrated and proved that all such organisms which essentially and
specifi-cally produce the enzyme
lecithinase e.g., Clostridium perfringens, on being
carefully grown on a solid egg-yolk medium, gives rise to well-defined colonies
usually surrounded by a zone of clearing.
15. Growth in Presence of Potassium Cyanide (KCN)
Occasionally,
buffered liquid-culture medium containing KCN in a final concentration of
ap-proximately 1/13,000 (i.e., 7.69 ×
10–5) is employed critically to identify certain KCN-tolerant enteric bacilli.
16. Composite Media
In the
domain of ‘Biochemical Tests’ the
pivotal role of composite media is
gaining legitimate recognition for the particular identification of biological isolates.
Advantages : The various cardinal advantages of the so called composite media are as enumer-ated
under :
·
it serves as an economical and convenient
culture media ; and
·
a ‘single
composite medium’ strategically indicates different characteristic
properties of the bacterium (under investigation) that otherwise necessarily
might have required the essential usage of several
individual cultural media.
Examples : The two most commonly employed
‘composite media’ are as described under :
(a) Triple Sugar Iron Medium (TSI-Medium) : It
represents a rather popular ‘composite
medium’ that specifically indicates
whether a bacterium under
investigation :
·
ferments glucose
exclusively,
·
ferments either, lactose or sucrose,
·
gas
formation occurs or not, and
·
indicates production
of H2S gas.
In actual
practice, TSI-medium is distributed
in various tubes along with a butt
and a slant. After having subjected them to proper innoculation under perfect
asceptic conditions one may draw the following inferences :
Red slant + Yellow butt. indicates
that all sugars viz., glucose, lactose,
and sucrose are duly fermented.
Appearance of bubbles in the butt—shows
production of gas, and
Blackening of the medium—displays
evolution of H2S gas in the
TSI-Agar Reaction.
Importance : The most spectacular and major
advantages of the TSI-medium is to
predomi-nantly facilitate the preliminary identification of the Gram-negative Bacilli.
(b) Test for Amino Acid Decarboxylation : The
specific biochemical test essentially involves the ‘decarboxylases’ (viz., arginine, lysine, ornithine) ; and
the phenomenon of decarboxylation of the amino acids invariably gives rise to
the corresponding release of amine
and CO2. In reality, this
particular test is solely employed for the identification
of enteric bacteria.
In
conclusion, one may add that there are certain other tests as well, namely ; fermentation of organic acids, hydrolysis of
sodium hippurate, and oxidation of
gluconate which are used some-times to carry out the identification of
certain critical organism(s). Now, with the advent of ever-increas-ing wisdom
and knowledge pertaining to the plethora of metabolic processes in the growth
of various microorganisms, the number of reliable tests also is increasing
progressively.
Note : One may consult the ‘special referred
manuals’ to have an access to the detailed descriptions as well as actual
utilities of these tests.
Biochemical Tests for Identification of Bacterial
Isolates : After having accomplished the microscopic and the critical growth characteristic features of a
pure culture of organisms are duly exam-ined ; highly precise and specific ‘biochemical tests’ may be carried out
to identify them exactly. Based on the survey of literature and genuine
evidences from various researches carried out one may come across certain ‘biochemical tests’ usually employed by
most clinical microbiologists in the
proper and methodical diagnosis of organism from the patients specimen.
A few
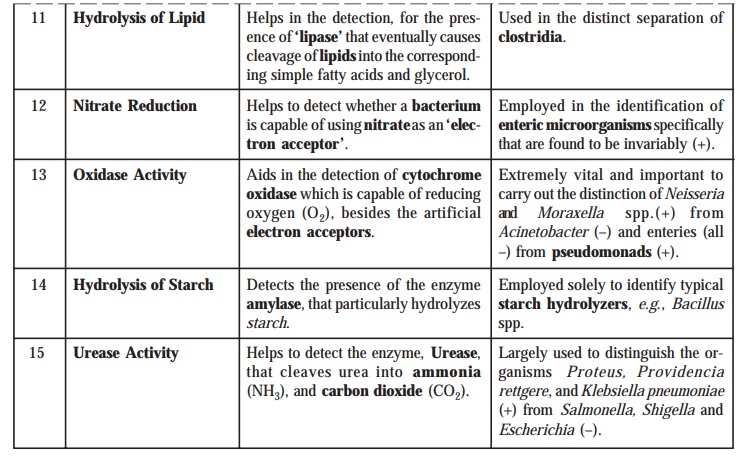
such typical examples are summarised duly in the following Table : 4.1.
Table : 4.1. Specific Biochemical Tests Carried out
by Clinical Microbiologists for the Critical Diagnosis of Microorganisms
Derived from the Patient’s Specimen Directly
Related Topics
