Catecholamines
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Adrenergic Drugs
Adrenergic : Catecholamines - Drugs Synthesis and Drug Profile - a. Adrenaline (Synonym: Epinephrine) b. Noradrenaline (Synonym: Norepinephrine, Nephridine) c. Dopamine (Domin, Dopacard) d. Isoprenaline (Synonym: Isoproterenol, Isoprim, Isosol, Neo-Epinin) e. Dobutamine (Cardiject, Dotamin, Kardia) - Structure, Properties, uses, Synthesis, Assay, Storage, Dosage forms, Dose | Synthesis and Drug Profile
SYNTHESIS AND DRUG PROFILE
Catecholamines
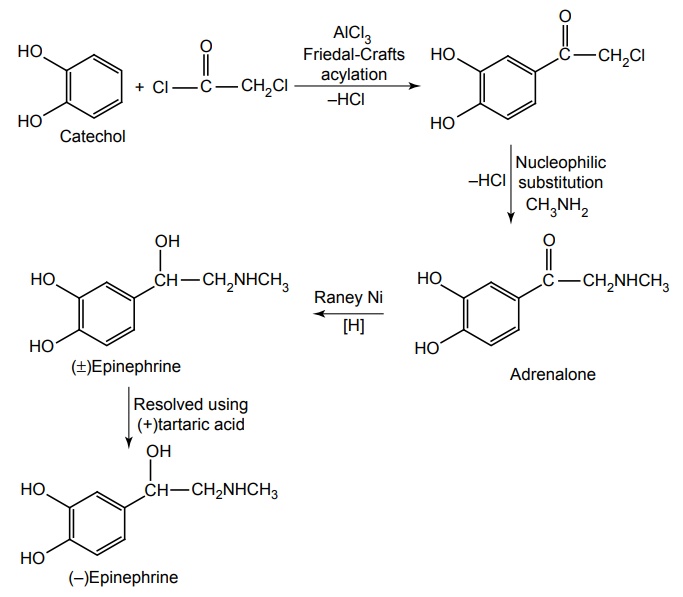
a. Adrenaline (Synonym: Epinephrine)
Synthesis
Metabolism of adrenaline and noradrenaline (catecholamines): Endogenous and exogenous catecholamines are metabolized by two enzymes monoamino oxidase (MAO) and catechol-O-methyl transferase (COMT). MAO converts catecholamines to their corresponding aldehydes, and in the periphery, the aldehydes are rapidly metabolized by aldehyde dehydrogenase to the corresponding carboxlic acid. In case of noradrenaline, this yields dihydroxy mandelic acid (DOMA). The second major pathway for catecholamine metabolism involves the methylation on one of the catechol –OH groups to give methoxy derivatives. O-Methylation of noradrenaline gives rise to a metabolite normetanephrine. Normetanephrine undergoes metabolism by MAO and 3-methoxy-4-hydroxy mandelic acid [vanillyl mandelic acid (VMA)] is formed, which is the main final metabolite of adrenaline and nonadrenaline.
Properties and uses: Adrenaline is a catecholamine and belongs to the family of biogenic amines. It is a white or creamy white, sphaero-crystalline powder. It dissolves in solutions of mineral acids, potassium hydroxide, and of sodium hydroxide, but sparingly soluble in water, insoluble in ethanol and ether. It is used as a sympathomimetic, broncholytic, and antiasthmatic. It is used to prevent bleeding during surgery or in case of inner organ bleeding. Because adrenaline leads to constriction of blood vessel, it is administered in combination with local anaesthetics. In this combination, anaesthetics have long-lasting effect and can be administered in smaller doses. It is used in the treatment of heart block or circulatory collapse and open-angle glaucoma. It is usually the drug of choice in acute allergic disorders and histamine reactions.
(R)-Epinephrine is 12 times more active than S form. It has a potent stimulatory effect on both α and β-adrenergic receptors and is light sensitive and easily oxidized on exposure.
Adrenaline is a potent stimulant for both α and β receptors, predominately on the β1 receptor of myocardium and pacemaker. The mechanism of rise in blood pressure is by
• direct myocardial stimulant (positive ionotropic action)
• increase in heart rate (positive chronotropic action)
• vasoconstrictor in the vascular beds.
Assay: It is assayed by nonaqueous titration, the solution of the substance is titrated with 0.1 M perchloric acid, using crystal violet indicator.
Storage: Epinephrine is light sensitive and easily oxidized on exposure to air because of the catechol ring system. The development of a pink to brown colour indicates oxidative breakdown. To minimize oxidation, solutions of the drug are stabilized by the addition of a reducing agent, such as sodium bisulphite. Adrenaline should be stored in well-closed airtight containers, which is preferably filled with nitrogen, and protected from light.
Dose: By subcutaneous, 0.2 to 0.5 mg in 0.1% solution; intramuscularly 1 to 3 mg in a 0.2% oil suspension, repeated as required.
Dosage forms: Adrenaline injection I.P., Adrenaline eye drops/epinephrine eye drops B.P., Dilute adrenaline injection (1 in 10,000)/dilute epinephrine injection (1 in 10,000) B.P.
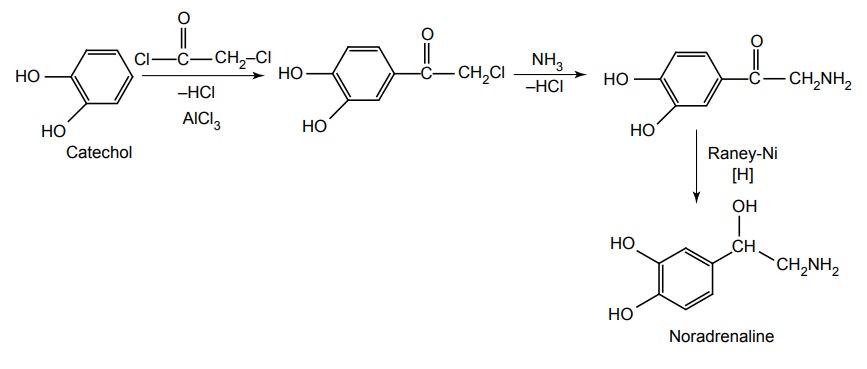
b. Noradrenaline (Synonym: Norepinephrine, Nephridine)
Synthesis
Properties and uses: It is a white or brownish-white, crystalline powder, slightly soluble in ethanol and soluble in water. It differs from adrenaline only by lacking the methyl substitution on the amino ethanol. L-isomer is pharmacologically active. Noradrenaline is a potent agonist for α receptors and has relative actions on β2 receptors. By acting on these receptors, the systolic and diastolic pressures, and usually, pulse pressure are increased. It increases the peripheral vascular resistance. Its principle use is to support blood pressure in various acute hypotensive states, especially in myocardial shock. It is used as a vasoconstrictor in some local anaesthetic solutions for dental use.
Assay: Dissolve the sample in acetic anhydride and add anhydrous formic acid. Titrate with 0.1 M perchloric acid and determine the end point potentiometrically
Storage: It becomes coloured on exposure to air and light. It should be stored in well-closed airtight containers, preferably in a sealed tube under vacuum or under an inert gas and protected from light.
c. Dopamine (Domin, Dopacard)
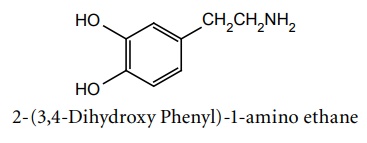
Synthesis
Route I. From: Veratrole
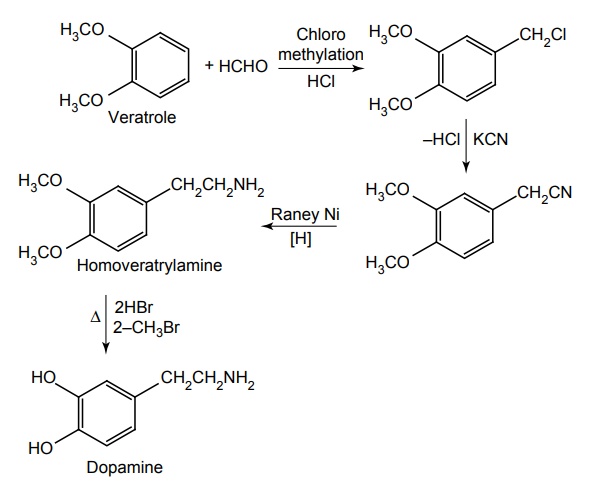
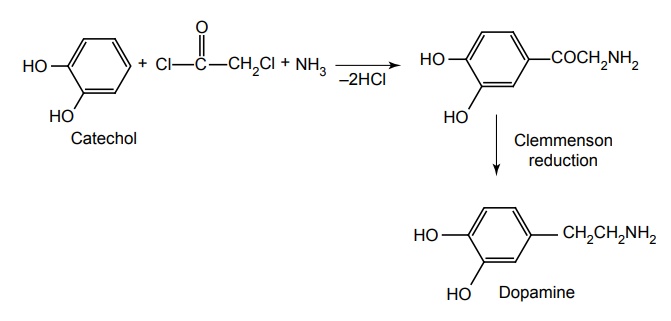
Route II. From: Catechol
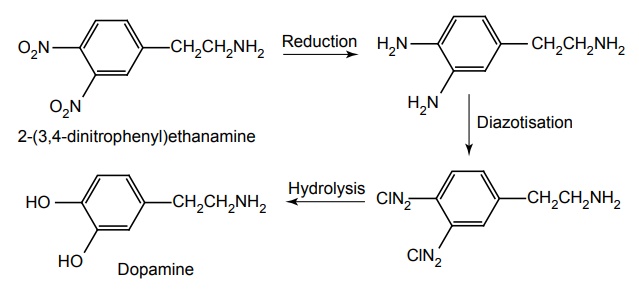
Route III. From: 2-(3, 4-dinitrophenyl) ethanamine
Properties and uses: It is a white or almost white crystalline powder, soluble in alcohol, sparingly soluble in acetone and methylene chloride, but freely soluble in water. It is used in the treatment of shock. It is ineffective orally in large parts because it is a substrate for both MAO and COMT. Dopamine exerts the CVS effects by interacting with D1-dopaminergic receptors especially in the renal, mesenteric, and coronary beds. At high concentrations, dopamine acts on β1 adrenergic receptors and causes positive ionotropic effects and also dopamine causes the release of norepinephrine.
Assay: Dissolve the sample in anhydrous formic acid and add acetic anhydride. Titrate with 0.1 M Perchloric acid and determine the end point potentiometrically
Storage: It should be stored in well-closed airtight containers, protected from light.
Dose: Acute heart failure: Adult: Initially, 1–5 μg/kg/min increased gradually by up to 5–10 μg/kg/min according to the patient’s BP, cardiac output and urine output. Up to 20–50 μg/kg/min may be required in seriously ill patients.
Dosage forms: Dopamine intravenous infusion B.P.
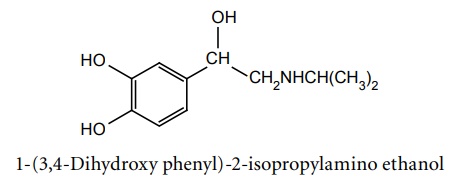
d. Isoprenaline (Synonym: Isoproterenol, Isoprim, Isosol, Neo-Epinin)
Synthesis
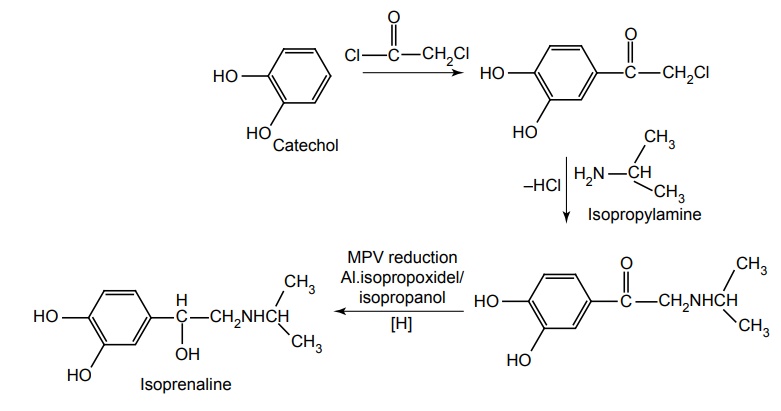
Metabolism: It is metabolized in the liver and other tissues by catechol-O-methyl transferase, which transfer the –CH3 group to the –OH group. It acts as poor substrate for MAO and it undergoes sulphate conjugation.
Properties and uses: It is a white or almost white crystalline powder, freely soluble in water, sparingly soluble in alcohol, practically insoluble in methylene chloride. It is a synthetic Isopropyl analogue of adrenaline, acting almost exclusively at β-receptor. It stimulates the action of adrenaline and has the advantage of being effective when given orally. It is a nonselective β agonist and has strong β1 and β2 agonist activity. Its primary use is in the treatment of bronchial asthma. It is used as an antiarrhythmic agent and in the treatment of shock to increase heart rate.
Assay: Dissolve the sample in anhydrous formic acid and add acetic anhydride. Titrate with 0.1 M perchloric acid and determine the end point potentiometrically.
Storage: It should be stored in well-closed airtight containers, protected from light.
Dose: Sublingual, 10 to 15 mg 3 to 4 times/day; I.M. or S.C. 0.01 to 0.2 mg; repeated as necessary; infusion, 1 to 2 mg per 500 ml of 5% dextrose infusion at such a rate so as to maintain blood pressure.
Dosage forms: Isoprenaline HCl injection I.P., Isoprenaline sulphate tablets I.P., Isoprenaline injection B.P.
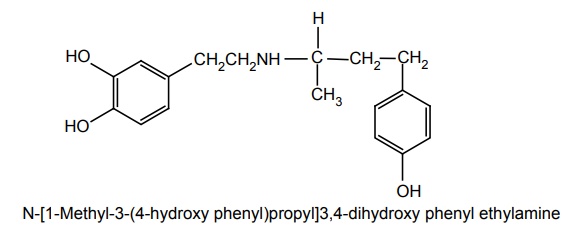
e. Dobutamine (Cardiject, Dotamin, Kardia)
Synthesis
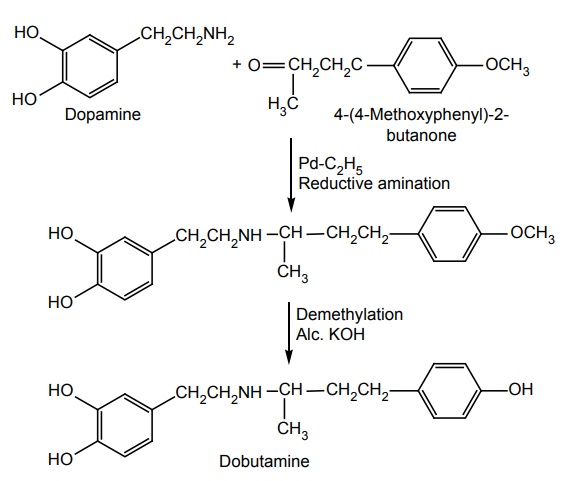
Metabolism: It is metabolized by COMT and conjugation, but not by MAO.
Properties and uses: It is a white or almost white crystalline powder, sparingly soluble in water and alcohol, and soluble in methanol. It resembles dopamine chemically, but possesses a bulky aromatic residue on the amino group despite the absence of a β-OH group. This substitution gives a compound that possesses an asymmetric carbon atom. Thus, dobutamine exists as a pair of enantiomers possessing a distinct pharmacology. The (+) enantiomer is a potent agonist at both β1 and β2 receptors. The (–) enantiomer is 10 times less potent at β1 and β2 receptors. The (–) enantiomer is a potent agonist at α receptors. It acts by directly interacting with α and β adrenergic receptors. Racemic dobutamine increases the inotropic action due to α1 receptor when compared to chronotropic actions, and the effects are mediated by β receptors. It enhances the automaticity of SA node.
Storage: It should be stored in well-closed airtight containers, protected from light.
Assay: Dissolve the sample in anhydrous formic acid and add acetic anhydride. Titrate with 0.1 M perchloric acid and determine the end point potentiometrically.
Dose: For acute heart failure: For adult: 2.5–10 μg/kg, up to 0.5–40 μg/kg according to patient’s heart rate, cardiac output, BP and urine output. For cardiac stress test: Adult: 5 μg/kg/min for 8 min using a 1 mg/ml solution, dose is then increased at 5 μg/kg/min until 20 μg/kg/min, with each dose being infused for 8 min before the next increase.
Dosage forms: Dobutamine intravenous infusion B.P.
Related Topics
