Noncatecholamines
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Adrenergic Drugs
Adrenergic Drugs : Noncatecholamines - Synthesis and Drug - a. Metaraminol (Aramine) b. Amphetamine (Synonym: Benzedrine) c. Salbutamol (Synonym: Albuterol, Asthalin, Salbid) d. Ephedrine (Epipres) e. Naphazoline (Ocucel Eye Dropa) f. Phenylephrine g. Isoxsupurine (Duvadilam, Suprox, Tidilan) h. Nylidrine HCl (Synonym: Arlidin) i. Xylometazoline (Synonym: Otrivin, Otrinoz, Decon) j. Terbutaline (Synonym: Bricanyl, Brethine, Asmaril) - Structure, Properties, uses, Synthesis, Assay, Storage, Dosage forms, Dose | Synthesis and Drug Profile
SYNTHESIS AND DRUG PROFILE
Noncatecholamines
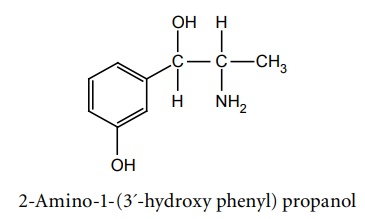
a. Metaraminol (Aramine)
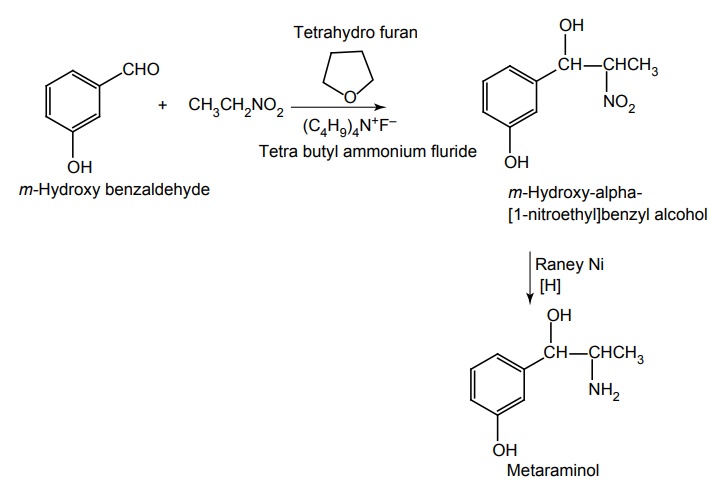
Synthesis
Route I. From: m-Hydroxy benzaldehyde
Route II. From: 1-(3-hydroxyphenyl)propane-1-one
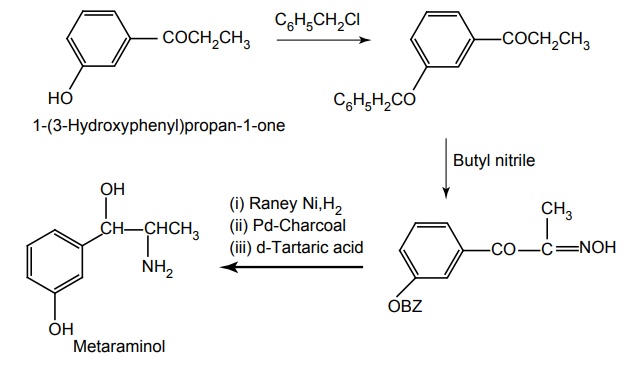
Properties and uses: It is a white crystalline powder, insoluble in ether, slightly soluble in ethanol, and freely soluble in water. It is structurally similar to phenylephrine. It enhances cardiac output, peripheral resistance, and blood pressure. It helps to increase the coronary blood flow thereby decreasing the heart rate. The drug is employed frequently in acute hypotensive states, such as anaphylactic shock or shock secondary to myocardial infarction and trauma.
Assay: It is assayed by nonaqueous titration: the solution of the substance is titrated with 0.1 M perchloric acid using crystal violet indicator.
Dose: By I.V. 0.5 to 5 mg in an emergency; by infusion, 15 to 100 mg/500 ml of dextrose injection or sodium chloride injection; By I.M. 2 to 12 mg.
Dosage forms: Metaraminol injection B.P.
b. Amphetamine (Synonym: Benzedrine)
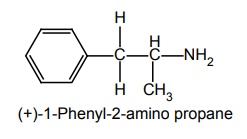
Synthesis
It is synthesized by reductive amination by phenyl acetone.

Properties and uses: The racemic mixture has a higher proportion of cardiovascular effects than the dextro isomer. For most medical uses, the dextrorotatory isomer is preferred. It is one of the most important sympathomimetic agents. CNS stimulant effect is due to stimulation of the cortex. The D-isomer is three to four times more potent than the L-isomer. It also has an anorexic action and can be used in the treatment of obesity.
c. Salbutamol (Synonym: Albuterol, Asthalin, Salbid)

Synthesis
Route I. From: Methyl-2-hydroxbenzoate
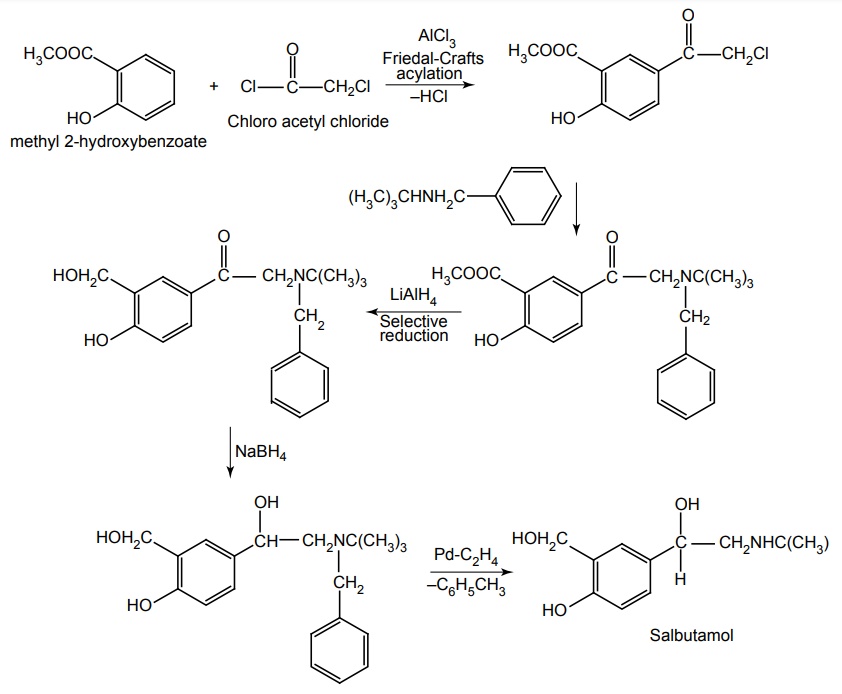
Route II. From: o-Hydroxy benzyl alcohol
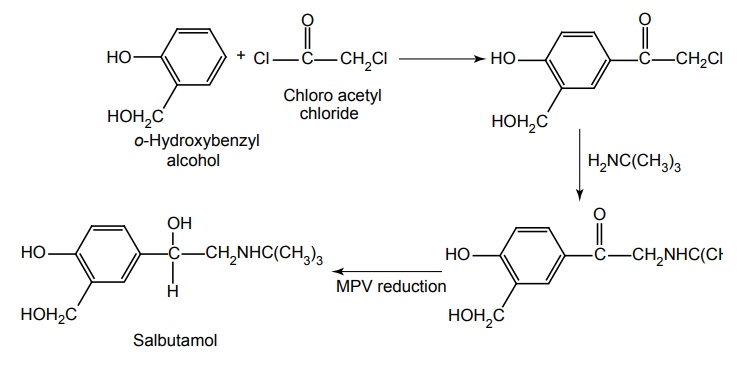
Properties and uses: It is a white or almost white crystalline powder, sparingly soluble in water, but freely soluble in ethanol. It has strong β adrenergic activity. It is useful in the treatment of acute myocardial infarction, severe left ventricular failure. It has been used to arrest premature labour and is effective in ocular hypotension by topical application. It is used only as a bronchodilator and is the drug of choice in the treatment of bronchial asthma.
Assay: Dissolve the substance in alcohol, to this add 0.1 M hydrochloric acid, titrate with 0.1 M sodium hydroxide using methyl red as indicator. End point is the appearance of yellow colour.
Storage: It should be stored in well-closed airtight containers, protected from light.
Dose: By oral inhalation the adult dose is 100 microgram, followed by a second dose after 5 min, if required.
Dosage forms: Salbutamol tablets and inhaler I.P., Salbutamol pressurized inhalation B.P.
d. Ephedrine (Epipres)
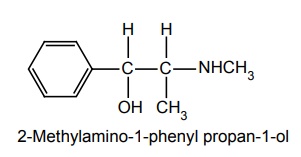
Metabolism: It is metabolized by COMT.
Properties and uses: Occurs as a waxy solid and as crystals, and has a characteristic pronounced odour. It is soluble in alcohol, water, and organic solvent. Ephedrine has two assymetric carbon atom and four optical isomers. The erythroracemate is called Ephedrine. It has both α and β-adrenergic agonistic effect. It is used in a variety of conditions, such as allergic disorder, colds, hypotension conditions, and narcolepsy. Also, occasionally, used to treat enuresis to dilate the pupil.
Synthesis

Assay: A sample is dissolved in alcohol and to this add 0.1N HCl. The solution is titrated with 0.1N NaOH, using methyl red as indicator.
Dose: The usual dose is 10 to 25 mg every 3 to 4 h.
e. Naphazoline (Ocucel Eye Dropa)
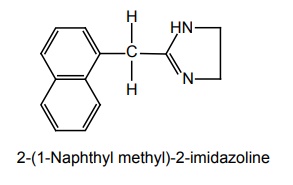
Synthesis
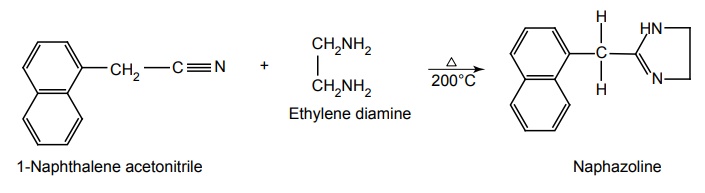
Properties and uses: It is a white crystalline, odourless, and bitter compound. The salt is soluble in water and in alcohol. They essentially exist in an ionized form at physiological pH because of the very basic nature of the imidazoline ring (pKa 9 to 10). It is a directly acting sympathomimetic drug, which is mostly used as a local vaso-constrictor for the relief of nasal congestion due to allergic or infarction manifestations. It is also employed as an ophthalmic solution for the relief of ocular congestion and blepharospasm.
Assay: Dissolve the sample in a mixture of 0.01 M hydrochloric acid and alcohol. Titrate with 0.1 M sodium hydroxide and determine the end point by potentiometric titration.
Storage: It should be stored in well-closed airtight containers, protected from light.
Dose: For nasal mucosa, 2 drops of 0.05% solution; for conjunctivity, 1 to 2 drops of a 0.1% solution after every 3 to 4 hours.
f. Phenylephrine
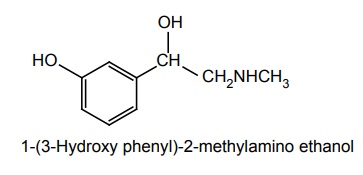
Synthesis
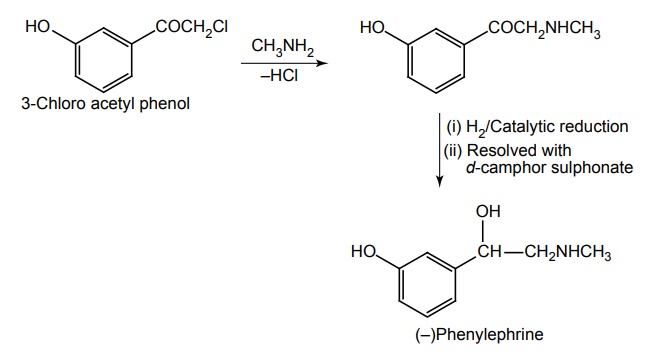
Route I. From: 3-Chloro acetyl phenol
Route II. From: Phenol
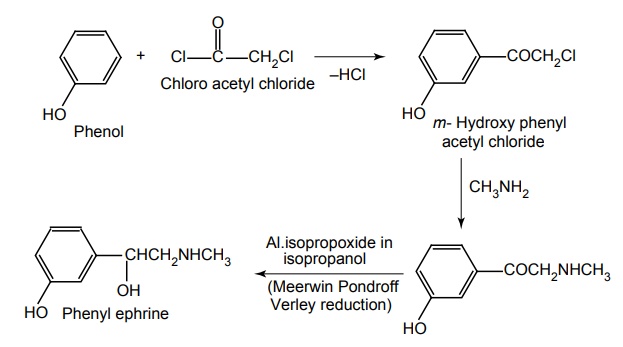
Properties and uses: It is a white or almost white crystalline powder, freely soluble in ethanol and water. Phenylephrine differs from adrenaline only by lacking the 4th OH group on the benzene ring, and subsequently, resistant to COMT and has predominantly α2 agonist effect. The L-isomer, causes marked arterial vaso-constriction and is active when given orally. It finds its main use in the relief of nasal congestion and as a mydriatic. It is also used to prolong the action of local anaesthetics.
Assay: Dissolve the sample in a mixture of 0.1 M hydrochloric acid and ethanol. Titrate with 0.1 M ethanolic sodium hydroxide and determine the end point by potentiometric titration.
Dose: By topical, intranasal, adults dose is 2 to 3 drops or 1 or 2 sprays of 0.2 to 0.5% solution; Intramuscular, adults, for mild to moderate hypotension: 2 to 5 mg repeated every 10 to 15 min.
Dosage forms: Phenylephrine eye drops B.P., Phenylephrine injection B.P.
g. Isoxsupurine (Duvadilam, Suprox, Tidilan)
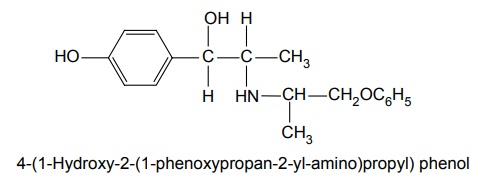
Synthesis
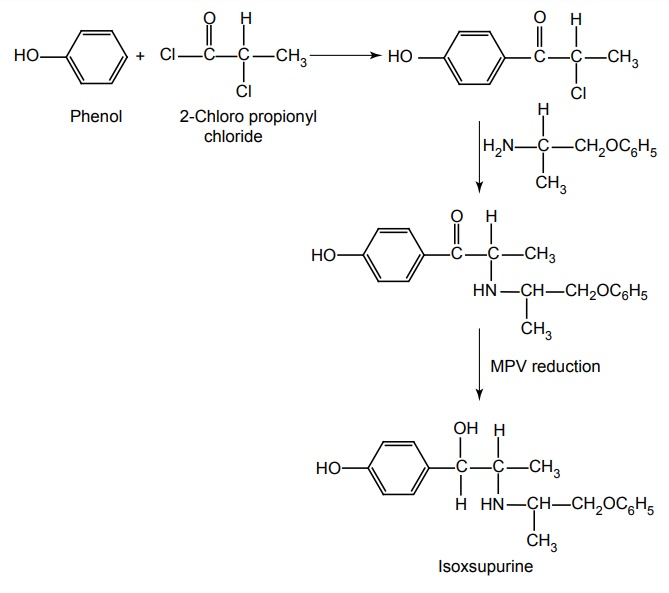
Properties and uses: It is a white or almost white crystalline powder, insoluble in methylene chloride, sparingly soluble in water and alcohol. It is used in the treatment of cerebral and peripheral vascular disease. It has also been employed in the treatment of Ménière’s disease and similar disorders of the internal ear.
Assay: Dissolve the sample in alcohol and add 0.1 M hydrochloric acid. Perform potentiometric titration using 0.1 M sodium hydroxide.
Storage: It should be stored in well-closed airtight containers and protected from light.
Dose: Orally 20 mg 4 times/day; I.V. infusion as a solution containing 100 mg in 500 ml of sodium chloride solution.
h. Nylidrine HCl (Synonym: Arlidin)
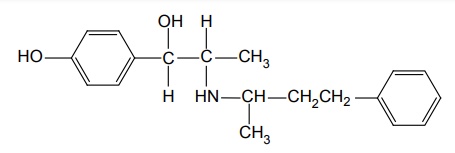
Synthesis
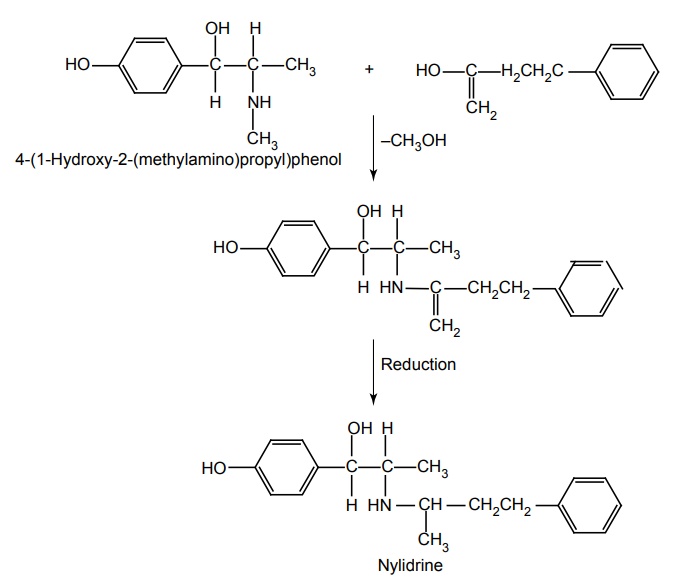
Dose: The usual dose initially by oral route 6 mg three times/day, which may be enhanced to 36 or 48 mg/ day in divided doses.
i. Xylometazoline (Synonym: Otrivin, Otrinoz, Decon)
Synthesis
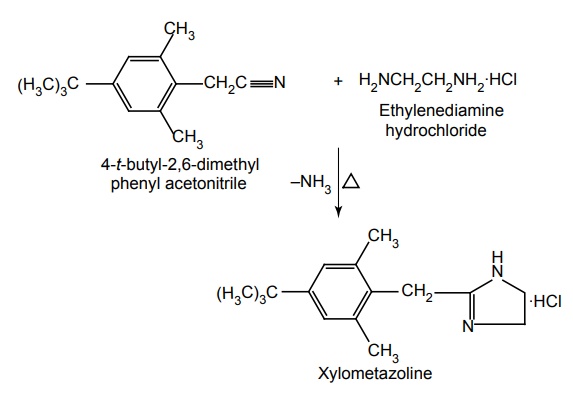
Properties and uses: It is a potent sympathomimetic agent, having marked and pronounced α-adrenergic pharmacologic profile. It is found to act as a vasoconstrictor, when applied topically to mucous membranes particularly. It is frequently employed as a local vaso-constrictor for nasal congestion caused by sinusitis or rhinitis.
Dose: By intranasal, 1 drop of a 0.1% solution in adult; or a spray of 0.05% solution.
j. Terbutaline (Synonym: Bricanyl, Brethine, Asmaril)

Properties and uses: It exists as a gray-white crystalline powder, odourless and with a bitter taste, soluble in water and alcohol. The drug exhibits the properties of a direct-acting sympathomimetic agent, having predominantly β adrenergic activity, and has a selective action on the β2 receptors (i.e. β2 agonist). It is used only as a bronchodilator and in the treatment of asthma. It possesses strong β-agonistic activity.
Synthesis
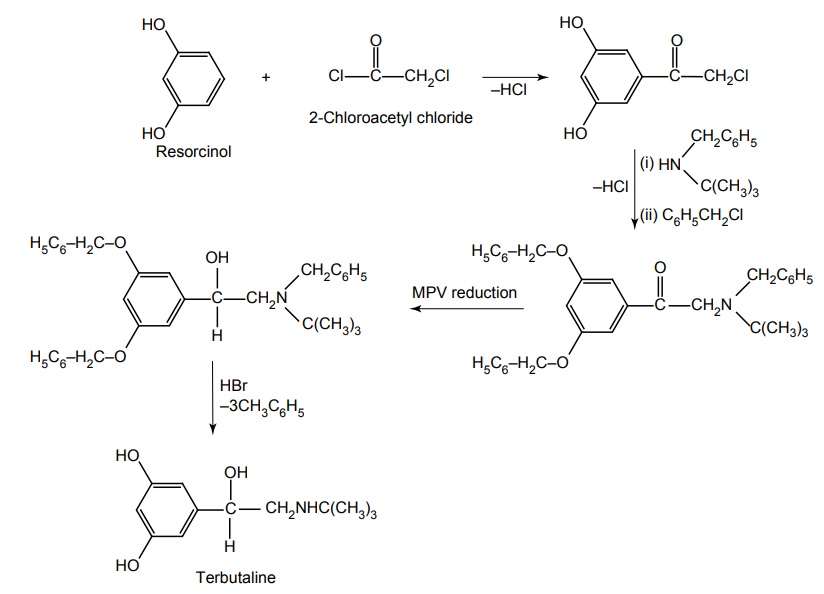
Dose: Uncomplicated premature labour: Adult 5mg/min for 20 min, increased every 20 min, steps of 2.5 μg/min until contractions have ceased (usually 10 μg/min sufficient), continue for 1 h, then decrease every 20 min in steps of 2.5 μg/min to lowest dose that maintains suppression, continue at this level for 12 h. Maximum dose is 20 μg/min. Switch to oral at 2.5–10 mg every 4–6 h, if indicated and tolerated. Continue as long as needed to prolong pregnancy.
Related Topics
