Characteristics of General Practice Research Database 2005
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: The General Practice Research Database
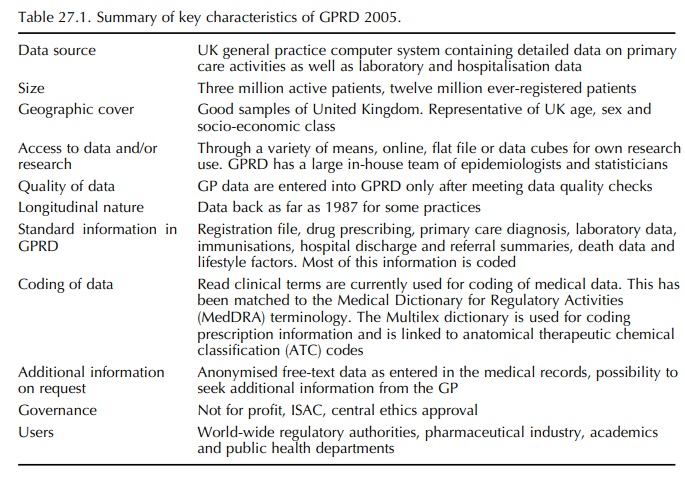
lists the main characteristics of the GPRD in 2005. It should be recognised that GPs use their computers primarily to create electronic medical records for the purpose of managing their patients.
CHARACTERISTICS OF GENERAL
PRACTICE RESEARCH DATABASE 2005
Table 27.1 lists the main characteristics of the GPRD in 2005. It should be recognised that GPs use their computers primarily to create electronic medical records for the purpose of managing their patients. However, contributing GPs are provided with record-ing guidelines that define what information should be recorded electronically so making the research under-taken in GPRD more valid:
• Demographics, including the patient’s age and
sex.
• Medical diagnosis, including free-text
comments.
• All prescriptions and immunisations as given
in primary care.
• Referrals to hospitals or specialists.
• Laboratory results, including microbiology.
• Treatment outcomes, including hospital
discharge reports where patients are referred to hospital for treatment.
• Key patient information, e.g. smoking status,
height and weight.
• Date and cause of death.
• Pregnancy-related information.
Following receipt and processing of a data collec-tion, the GPRD Group provide feedback reports to the contributing practice on the completeness of data in key areas (e.g. date and cause of death and patient registration details), to enable practices to address any deficiencies they have with their recording. In addi-tion, the quality of recording across the entirety of data contributed by a practice is assessed by means of the ‘up to standard’ audit that assesses the complete-ness, continuity and plausibility of data recording in key areas, in accordance with the recording guide-lines issued to practices. Where data quality is found to be acceptable, the practice is judged to be ‘up to standard’ and marked as such in the database; this marker can be used to identify those practices where data recording is considered by the GPRD Group to be of sufficient quality for research purposes.
In
April 2004, the Quality Outcomes Framework (QOF) was introduced into UK general
practice. This framework provides incentives to practices for the provision of
high quality care that naturally involves improved data documentation. Data
from the prac-tice records are submitted to and analysed by the Quality
Management and Analysis System (QMAS), a national IT system, that supports the
QOF payment process. Achievement is measured against indica-tors in four
domains; most importantly, the clinical domain, which focuses on a range of
indicators in 11 key disease areas. Given the key role of practice records in
supplying the data needed to assess prac-tice achievement/performance, there is
now even more emphasis for practices to ensure that their records are complete,
particularly in areas related to QOF indica-tors. This can only be of benefit
to research.
The
GPRD is a unique public health research tool, which has been used widely for
drug safety stud-ies and many other types of pharmacoepidemiolog-ical research.
There are now over 500 GPRD-based peer-reviewed publications, nearly 2000
peer-review impact points and an ever expanding international user base.
Numerous independent validation studies have confirmed a high level of
completeness and validity of the data in the GPRD. A large study recently
examined the validity of the computerized diagnoses of autism in the GPRD.
Anonymised copies of all relevant avail-able clinical reports, including GP’s
notes, consultant, speech therapy and educational psychologists reports, were
evaluated for 318 subjects with a diagnosis of autism recorded in their
electronic general prac-tice record. For 294 subjects (92.5%), the diagnosis of
pervasive developmental disorder was confirmed after review of the records, providing
evidence that the positive predictive value of a coded diagnosis of autism
recorded in the GPRD is high (Fombonne et
al., 2004). Another study compared the distribution of the cause of death in GPRD to national mortality statistics and
concluded that they were broadly simi-lar. This provides further evidence that
the GPRD population is broadly representative of the general population (Shah
and Martinez, 2004).
Recent
research includes a case-series analysis of the risks of myocardial infarction
and stroke after common vaccinations and naturally occurring infec-tions. It
found that there was no increase in the risk of myocardial infarction or stroke
in the period after influenza, tetanus or pneumococcal vaccination. However,
the risks of both events were substantially higher after a diagnosis of
systemic respiratory tract infection and were highest during the first 3 days,
suggesting that acute infections are associated with a transient increase in
the risk of vascular events (Smeeth et al.,
2004). A study by Martinez compared the risk of non-fatal self-harm and suicide
in patients n taking selective serotonin reuptake inhibitors (SSRIs) with that
of patients taking tricyclic antidepressants. No evidence was found that the
risk of suicide or non-fatal self-harm in adults prescribed SSRIs was greater
than in those prescribed tricyclic antidepres-sants (Martinez et al., 2005).
Most
of the drug safety research in the GPRD has concerned the estimation of
relative rates, i.e. the rate of outcomes in exposed patients divided by that
in control patients. But relative rates do not convey the public health
importance of a safety issue. Large rela-tive rates for rare events may not be
of major concern, whereas small relative rates for frequent events may
potentially have large implications. An example for this may be the
cardiovascular risk of selective cyclooxygenase-2 inhibitors, which may have
affected a large number of patients. Recent research developed methods to
estimate, in the GPRD, individual long-term probabilities specific for a
patient’s age, sex and clinical characteristics. This was done for estimating
the long-term risk of fracture in patients using oral glucocorticoids. As an
example, it was found that a woman aged 65 years with rheumatoid arthritis, low
body mass index (BMI) and a previous history of frac-ture and falls, who used
15 mg glucocorticoids daily, would have a 5-year fracture risk of 47% (a man
with similar history, 30.1%) (van Staa et
al., 2005). This approach to quantify individualised long-term
probabilities can help to better quantify the risks and benefits associated
with a treatment.
Related Topics
