Current and Future Developments in General Practice Research Database
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: The General Practice Research Database
The data in the GPRD is a person-level data set with the linkage to other information currently undertaken within the primary care system by the GP and his staff.
CURRENT AND FUTURE DEVELOPMENTS
IN GENERAL PRACTICE RESEARCH DATABASE
The
data in the GPRD is a person-level data set with the linkage to other
information currently undertaken within the primary care system by the GP and
his staff. This is, in many ways, the ideal situation as the GP, or other
primary care healthcare professionals, do the disease coding at the time of
consultation. Increas-ingly, the information within the UK NHS is being
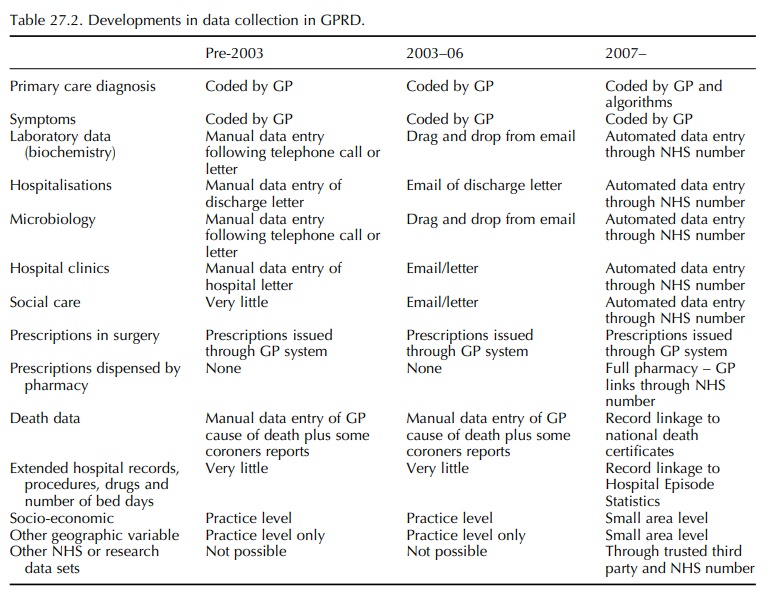
communicated electronically using the NHS number unique for each patient. Table
27.2 lists the major changes in GPRD data collection that are happening.
Laboratories are now sending biochemistry results electronically to the GP, and
this information can be loaded electronically into the GP medical records. Over
the years 2002–05, the amount of biochem-istry data in the GPRD has increased
three fold due to increases in the number of tests undertaken, the fact that
tests are grouped for common requirements even when only one result was
requested and auto-matic recording of these data (with better recording of results
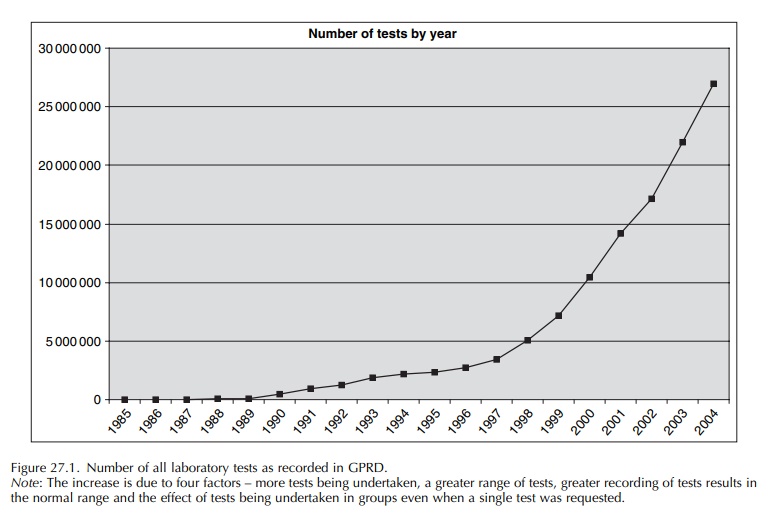
within normal reference ranges). Figure 27.1 shows the number of laboratory
results as recorded in the GPRD over calendar year. It is likely that the
introduction of the QOF has also directly impacted on the recording of tests in
the GPRD. The frame-work focuses on key disease areas such as diabetes,
hypertension and asthma; it is expected that specific tests are conducted (and
the results recorded in the electronic record) in patients with diagnoses in
these key disease areas. For instance, diabetic patients are expected to have a
record of HbA1c or equivalent in the previous 15 months. Recording of HbA1c in
2004 was 13% higher than in 2003; the requirement under QOF to measure and
record HbA1c levels for diabetic patients makes it likely that the increase in
record-ing is not simply because of the general increase in recording of test
results in patient records resulting from the electronic transmission of test
results.
The
quality of GPRD recording of lifestyle factors such as weight, BMI, smoking and
alcohol use is continuously improving and is not as reported by Ilkanoff et al. (2005) a limitation to using
GPRD. The reason is that NHS has undertaken initiatives to improve data recording
in GP practices and has linked quality standards in care and data recording to
practice reimbursement. The GPRD currently only records prescriptions as
written by the GP, but due to NHS IT initiatives, data from pharmacies on
dispensed drugs may also become available over the coming years.
As
of 2007, the GPRD will be using a trusted third party to enable record linkage
to other NHS data sets. This linkage is planned for practice-level
socio-economic class and complete death certificate information. It will be
done at regular intervals and available to all researchers. Other linkages will
only be available subsequent to ethics and scientific proto-col approval and
only for that specific study as these linkages bring additional requirements
for governance and data privacy. Detailed information on hospitalisa-tions
(including main procedures and number of bed days) may also become available.
The following programmes have been developed to allow even better use of the GPRD:
·
Risk-management
programmes:
Pharmaceutical companies are now
required to submit Risk-Management Plans to regulatory authorities for newly
approved drugs, dose changes and new indications. Systematic data
collection on a large cohort of drug users in routine clini-cal practice is an
important element of risk management. The GPRD Group has developed the Risk
Management Knowledge and Track-ing programme, which allow the monitoring of
outcomes in drug users and importantly the key background information required
for case assessment.
·
Surveillance
programmes:
Patients prescribed a drug can be
followed for selected outcomes. Further information (including hospitalisation
records) may then be requested. This information can then be used to assess the
causality of the individual cases and also to estimate overall risks.
·
Randomised
simplified trials: Subject to appro-priate approval (including the patient’s
informed consent and approval by an ethics committee), it will be possible in
selected practices to randomise patients to various treatments. Patients can
then be followed using routine data collection to evaluate the outcomes.
Confounding by indication is a major concern in pharmacoepidemiological
research, and this randomisation can overcome bias due to baseline differences.
· Prospective data collection: Subject to appropriate approval (including the patient’s informed consent and approval by an ethics committee), additional information can be obtained through the GP. This can include genetic samples. Pharmaco-genetic studies could be conducted to evaluate the effect of genetic polymorphisms on the response of drug treatment.
Current research governance guidance is to separate the
scientific and ethical elements of protocol review, and the GPRD Group is
currently working to implement new plans that will put its research governance
arrangements on a robust footing with regard to current best practice. From
March 2006, the Independent Scientific Advisory Committee (ISAC) for MHRA
database research will be responsible for the scientific review of protocols
for research using GPRD data. Members of this independent committee are
appointed following a formal recruitment exer-cise run by the NHS Appointments
Commission. The committee membership includes expert epidemi-ologists and
statisticians as well as GPs and a lay member. Whilst the committee’s remit
with regard to protocols is confined to the scientific aspects of the proposed
research, it will have the ability to refer protocols for further ethical
review by an NHS Research Ethics Committee (REC) where the proposed research is
not covered by the existing ethics approval.
Related Topics
