Chelating Agents
| Home | | Pharmacology |Chapter: Essential pharmacology : Chelating Agents
These are drugs which complex metallic ions, forming ring structures within their molecule (Greek Chele = Crab; the compound holds the metal like a crab’s claw). They are primarily used in heavy metal poisonings.
CHELATING AGENTS
These are drugs which
complex metallic ions, forming ring structures within their molecule (Greek Chele = Crab; the compound holds the
metal like a crab’s claw). They are primarily used in heavy metal poisonings.
Those compounds which form stable, nontoxic and easily excreted
complexes with toxic metals are valuable in poisonings. The useful agents
contain two or more reactive groups (ligands) which can hold the metal from at
least two sides so that a ring is formed. When the ring is 5–7 membered, it is
most stable.
Ligand is a functional group
capable of forming coordinate bond,
i.e. a covalent bond in which both the shared electrons are donated by the ligand—generally
O, N, or S atoms in hydroxyl, carboxyl, keto, sulfhydryl, disulfide, amino or
phosphate groups.
Heavy metals exert their toxic effects by combining with and
inactivating functional groups (ligands) of enzymes or other critical biomolecules.
Chelating agents compete with body ligands for the heavy metal. They differ in
their affinity for different metals. Clinically useful agents should have a
higher affinity for the toxic metal than for calcium, because Ca2+ is readily available
in plasma and extracellular fluid. They should also have higher affinity than
the body ligands for the toxic metal. Moreover, to be effective in metal
poisoning, their distribution in the body should correspond to that of the
metal to be chelated, and they should be water soluble.
Efficacy of all chelating agents in promoting excretion of the
toxic metal and in reversing toxic manifestations declines rapidly as the
interval between entry of the metal in the body and the administration of the
chelator increases.
Chelating agents useful as drugs are:
Dimercaprol (BAL)
Calcium disodium DTPA
Dimercaptosuccinic Penicillamineacid (Succimer)
Desferrioxamine
Disodium edetate
Deferiprone
Calcium disodium edetate
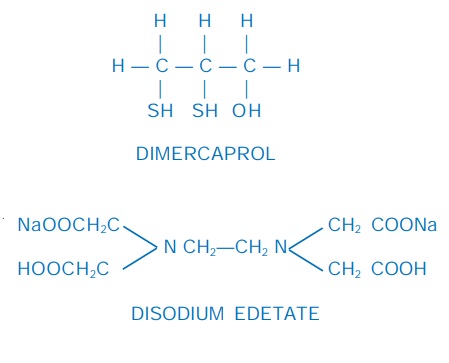
Dimercaprol (British anti-lewisite; BAL)
It is an oily, pungent smelling, viscous liquid, developed
during World War II by Britishers as an antidote to the arsenical war gas lewisite. The two SH groups of
dimercaprol bind those metals which produce their toxicity by interacting with
sulfhydryl containing enzymes in the body, i.e. As, Hg, Au, Bi, Ni, Sb, Cu. The
complex of 2 molecules of dimercaprol with one metal ion is more stable than
1:1 complex. It is, therefore, desirable to maintain excess of dimercaprol in
plasma to allow formation of 2 : 1 complex. The dimercaprol-metal complex spontaneously
dissociates releasing the metal at a slow rate; also dimercaprol is partly
oxidized in the body: further emphasizing the necessity to have excess
dimercaprol available. But due to dose dependent toxicity of dimercaprol, large
amounts should not be given at a time.
Uses
Poisoning by As, Hg,
Au, Bi, Ni, Sb: it is administered i.m., 5 mg/kg stat, followed by 2–3 mg/kg every 4–8 hours for 2 days, then once
or twice a day for 10 days. It is partly oxidized and glucuronide conjugated,
but mainly excreted as such in 4–6 hours. Earlier the treatment is instituted,
the better it is. Because the dimercaprol-metal complex dissociates faster in
acidic urine and the released metal can damage the kidney, urine is alkalinized
during dimercaprol therapy.
As an adjuvant to Cal. disod. edetate in lead poisoning.
As an adjuvant to penicillamine in Cu poisoning and in Wilson’s
disease—300 mg/day i.m. for 10 days every second month.
BAL INJ 100 mg/2 ml
(in arachis oil) inj.
It is contraindicated in iron and cadmium poisoning, because the
dimercaprolFe and dimercaprolCd complex is itself toxic.
Adverse effects
These are frequent,
dose related and distressing, but
generally not damaging. Rise in BP, tachycardia, vomiting, tingling and burning
sensations, inflammation of mucous membranes, sweating, cramps, headache and anxiety.
Antihistaminics given
30 min before dimercaprol injection, reduce the intensity of adverse effects.
Dimercaptosuccinic acid (Succimer)
It is similar to dimercaprol in chelating properties, water
soluble, less toxic and orally effective. Its efficacy has been demonstrated in
As, Hg and Pb poisoning. It has been marketed in USA and some other countries,
but not in India for the treatment of lead intoxication. Side effects are
nausea, anorexia and loose motions.
Disodium edetate (Na2EDTA)
It is the disodium salt
of ethylene diamine tetraacetic acid (EDTA). It is a potent chelator of
calcium—causes tetany on i.v. injection. When a slow infusion is given, tetany
does not occur, because calcium is withdrawn from bones. It can be used for
emergency control of hypercalcaemia: 50 mg/kg i.v. infusion over 2–4 hours, but
bisphosphonates are preferred.
Calcium disodium edetate (Ca Na2 EDTA)
It is the calcium
chelate of Na2 EDTA. Because this chelating agent has higher affinity
for metals like Pb, Zn, Cd, Mn, Cu and some radioactive metals, it can remove
them from the body by exchanging with Ca held by it. It is highly ionized, therefore
distributed only extracellularly and rapidly excreted in urine by glomerular
filtration (t½ < 1 hour) carrying the toxic metal along. It is not
metabolized. Because of its ionic nature, Ca Na2 EDTA is not absorbed
from the g.i.t.—must be given parenterally. Since i.m. injection is painful, preferred
route is i.v. It does not enter brain or CSF. Thus, it can remove toxic metals
only from accessible sites.
Uses
Lead
Poisoning
This is the most important indication for CaNa2EDTA; 1 gm is diluted to 200–ml in saline or glucose solution and infused i.v. over 1 hour twice daily for 3–5 days. The urinary excretion of Pb is promptly increased, but declines quickly as the metal is removed from accessible sites (primarily bone). A second course of CaNa2EDTA may be repeated after 5–7 days, allowing time for Pb to redistribute to extracellular sites.
It is also useful in Fe, Zn, Cu, Mn and radioactive metal, but
not Hg poisoning, because Hg is more firmly bound to body constituents and is
localized in areas not accessible to CaNa2 EDTA.
Adverse Effects CaNa2 EDTA does not produce tetany and is relatively safe.
Kidney damage with proximal tubular necrosis is the most
important problem. This is roughly dose-related and may be due to the toxic
metal partly dissociating in the tubule. It can be minimized by maintaining high
urine flow.
An acute febrile reaction with chills, body-ache, malaise,
tiredness occurs in some individuals. Anaphylactoid reaction with fall in BP
and congestion of eyes and nose is also reported.
Calcium disodium DTPA
Diethylene triamine
penta acetic acid (DTPA, Pentetic
acid) is a congener of EDTA. It has higher affinity for many heavy metals than
EDTA. Its calcium chelate has been used in metal poisonings (especially radioactive
metals like urenium, plulonium) which do not respond to CaNa2EDTA.
However, because of its limited distribution in the body, results are not
impressive.
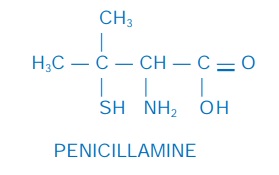
Penicillamine
It is dimethyl cysteine, obtained as a degradation product of
penicillin. It was found to have strong copper chelating property and was used
in 1956 for Wilson’s disease. It selectively chelates Cu, Hg, Pb and Zn. The disomer
is used therapeutically, because the lisomer and the recemate produce optic
neuritis and are more toxic. It is adequately absorbed after oral
administration, little metabolized in the body and excreted in urine and
faeces. When given to patients with heavy metal toxicity, excretion of the
metal is enhanced.
Uses
Wilson’s disease (Hepatolenticular degeneration): This is due to
genetic deficiency of ceruloplasmin, a protein which normally binds and
disposes off Cu from the body. In its absence, plasma concentration of free Cu
is high which gets deposited in liver, substantia nigra, basal ganglia of
brain, and causes local degeneration. Life long therapy is needed to prevent
progression of the disease.
Dose: 0.5–1 g daily in
divided doses 1 hour before or 2 hour
after meals to avoid chelation of dietary metals. ARTAMIN, CILAMIN 250
mg cap, ARTIN 150, 250 mg cap.
Pot. sulfide 20–40 mg may be given with each meal to decrease
the absorption of dietary copper.
Copper/mercury poisoning: 1–1.5 g/day is given
for a few days. It is the drug of choice for Cu poisoning and alternative drug
to dimercaprol/ succimer for Hg poisoning.
Chronic lead poisoning: It may be used as an
adjuvant to CaNa2EDTA, but succimer is preferred.
Cystinuria and cystine stones: It promotes the
excretion of cysteine and prevents its precipitation in the urinary tract,
because penicillaminecysteine complex is more soluble than dicysteine
(cystine).
Scleroderma: Penicillamine
benefits by increasing soluble collagen.
It was used as a disease modifying drug in rheumatoid arthritis,
but has been replaced now by safer drugs .
Adverse Effects
Short-term administration (as metal chelator) of penicillamine
does not cause much problem. Various cutaneous reactions, itching and febrile
episodes may occur. However, long-term use produces pronounced toxicity. Dermatological,
renal, haematological and collagen tissue toxicities are prominent.
Desferrioxamine
Ferrioxamine is a long chain iron containing complex obtained
from an actinomycete. Chemical removal of iron from it yields desferrioxamine
which has very high affinity for iron: 1g is capable of chelating 85 mg of
elemental iron. The straight chain desferrioxamine molecule winds round ferric
iron and forms a stable nontoxic complex which is excreted in urine. It removes
loosely bound iron as well as that from haemosiderin and ferritin, but not from
haemoglobin or cytochrome. Another desirable property is its low affinity for
calcium.
Little of orally administered desferrioxamine is absorbed.
Parenterally administered desferrioxamine is partly metabolized and rapidly
excreted in urine.
Uses
Acute iron poisoning: mostly in children. This is the most
important indication—may be life saving .
Transfusion siderosis: occurs in thalassemia patients who receive
repeated blood transfusion. Desferrioxamine 0.5–1 g/day i.m. helps to excrete
the chronic iron overload; may also be infused i.v. concurrently with blood
transfusion—2 g per unit of blood.
Adverse effects
Desferrioxamine can
cause histamine release → fall in BP, flushing,
itching, urticaria, rashes. A variety of allergic reactions are reported.
Changes in lens and retina can occur on repeated use.
Other side effects are
abdominal pain, loose motions, muscle cramps, fever and dysuria.
DESFERAL 0.5 g/vial
inj.
Deferiprone
It is an orally active
iron chelator which has simplified the treatment of transfusion siderosis in
thalassemia patients. Excessive haemolysis occurs in these patients, and they
have to be given repeated blood transfusions. An iron chelator has to be used
to clear the resulting iron overload. Oral deferiprone is a somewhat less
effective alternative to injected desferrioxamine. Side effects and cost of
treatment are reduced. Deferiprone has also been indicated for acute iron
poisoning (less effective than desferrioxamine) and for iron load in liver
cirrhosis.
Dose: 50–100 mg/kg daily in
2–4 divided doses.
KELFER 250, 500 mg
caps.
Side Effects are anorexia, vomiting, altered taste, joint pain, reversible neutropenia,
rarely agranulocytosis. However, long-term safety is not yet known.
Related Topics
