Epidemiology
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Hepatic ADRs
The epidemiology of adverse hepatic reaction remains poorly documented. Controlled clinical trials have the advantages of close and prospective surveillance as well as a control group.
EPIDEMIOLOGY
METHODS OF ESTIMATING THE FREQUENCY OF ADVERSE HEPATIC REACTIONS
The
epidemiology of adverse hepatic reaction remains poorly documented. Controlled
clinical trials have the advantages of close and prospective surveillance as
well as a control group. However, the median number of subjects exposed to a
new drug at the time of marketing is usually around 1500, with rarely more than
100 patients receiving the product for more than a year (Rawlins, 1995). This
is clearly inade-quate as around 30 000 treated subjects need to be observed to
identify, with a power 0.95, at least one with drug hepatotoxicity when the
incidence is 1 in 10 000 patient years (Stricher, 1992). The debate surrounding
the initial approval and the recent with-drawal from the market of troglitazone
highlights the realities of the drug development and the need for
post-marketing surveillance. In the clinical trials of troglitazone
(representing a novel class of oral anti-hyperglycaemic agents), 1.9% of
patients receiving the drug had elevated liver enzymes, two of which developed
reversible jaundice (Watkins and Whit-comb, 1998). It took more than 3 years
and 90 deaths or liver transplantation (in over a million patients treated),
before the drug was withdrawn from the market (Lumpkin, 2000). Furthermore,
clinical trials usually include selected patients, and the findings may
therefore not be generalised to a wider popula-tion. Hence, in the United
Kingdom and many other countries, post-marketing surveillance relies largely on
spontaneous reporting (Rawlins, 1995), and data on adverse hepatic reactions
have come most often from this source. Spontaneous reporting allows the
surveillance to continue throughout the life of the marketed drug when a large
number of individu-als have been exposed to the drug, and hence rela-tively
rare adverse reactions have been recognised. However, only 10% of the serious
and 2%–4% of non-serious reactions are usually reported (Rawlins, 1995). A
relatively high rate of reporting may result from a high frequency of adverse
reactions or may simply be because of the publicity or novelty of a new agent.
One such ‘apparent epidemic’ of flucloxacillin-induced jaundice in Australia
(reporting 357 ADRs and 17 deaths) has been considered to be a reporting
artefact (Devereaux et al., 1995;
Roughead, Gilbert and Primrose, 1999). In addition to the variability of
reporting, the identification of cases in a non-systemic way introduces
significant inaccuracy to the data. In a recent survey in the United Kingdom,
about half of the reported adverse hepatic reactions were classified as
‘unrelated’ to the drugs under systemic evaluation (Aithal, Rawlins and Day,
1999). A further difficulty with spontaneous reporting is that the denominator
is usually unknown, although drug sales figures could be used to estimate the
frequency of adverse reactions.
Recording
linkage studies connect information on drug exposure from prescription data
with outcome and have the advantages of prospective design and comprehensive
identification of cases. Established linkages such as the General Practice
Research Database in the United Kingdom and Group Health Cooperative of Puget
Sound in the United States have contributed valuable epidemiological
informa-tion regarding drug-induced liver disease (Beard et al., 1986; Derby et al.,
1993; Jick, Stender and Myers, 1999). However, most often the outcomes such as
deaths, hospital admissions and discharge diagnoses, used in linkage studies,
are those that pertain only to the more serious reactions or those that occur
while the patient is in the hospital. The latter underestimates the frequency
of adverse hepatic reactions as acute hospital inpatient stays are usually
shorter than the latent period (5 days to 3 months) of most types of
drug-induced liver disease.
Case–control
studies are particularly useful when the outcome is rare. In the field of
drug-induced liver disease, they have been applied to hepatic tumours,
industrial hepatotoxicity and the role of aspirin in Reye’s syndrome (Farrell,
1994).
FREQUENCY OF ADVERSE HEPATIC REACTIONS
Despite
increasing awareness of hepatotoxicity and the availability of less toxic
alternatives, the absolute frequency of hepatic drug reactions has not
decreased in the last decade, in keeping with the increasing number of
prescriptions and pharmacological agents available (Larrey, 2000). Hepatic
injury accounts for 3.5%–9.5% of all ADR reports and up to 14.7% of fatal
adverse reactions (Friis and Andreasen, 1992; Aithal, Rawlins and Day, 1999). A
recent prospective population-based study from France suggested that the number
of hepatic ADRs would be 16 times greater than that reported to the regulatory
authorities (Sgro et al., 2002). In
this study, the global crude annual incidence
rate was 14 per 100 000 population, and the standardised annual incidence rate
was estimated to be 8.1 per 100 000 population (Sgro et al., 2002). Acute serious liver injury requiring hospitalisation
has been estimated to be 7–10 per 1 000 000 population per year (Ibanez et al., 2002; Sgro et al., 2002).
THE CONTRIBUTION OF DRUG-INDUCED HEPATOTOXICITY TO THE OVERALL BURDEN OF LIVER DISEASE
Drugs
are responsible for between 2% and 6% of jaun-dice and about 10% of cases of
‘acute hepatitis’ (Lewis and Zimmerman, 1989; Whitehead, Hainsworth and
Kingham, 2001). In industrialised nations such as the United States, ADRs
account for up to 13% of cases of acute hepatic failure, while it is less
common (5%) in tropical countries such as India (Acharya et al., 1996; Ostapowicz et
al., 2002). Drug-induced chronic hepati-tis has been considered rare, even
though it accounts for up to 6% of all chronic hepatitis (Aithal and Day,
1999). Drug hepatotoxicity almost certainly remains an important and often
neglected cause of cholestasis, although its relative frequency among other
cholestatic syndromes has not been reported. Drugs probably contribute to the
aetiology of less than 1% of all liver tumours (Farrell, 1994).
RELATIVE FREQUENCIES OF DRUGS IMPLICATED
Advances
in drug development have allowed the replacement of many potentially toxic
drugs with ‘safer’ alternatives. For example, oxyphenisatin has been withdrawn
as a laxative in most countries, alpha-methyldopa is now rarely used as an
antihyperten-sive agent, and alternative, safer agents have replaced
perhexiline. As might be expected, this has led to a change in the pattern of
implicated drugs caus-ing hepatotoxicity over the last four decades. In the
1960s, chlorpromazine was most commonly associ-ated with hepatotoxicity (Cook
and Sherlock, 1965), and in the 1970s, halothane continued to account for
significant numbers of hepatotoxic adverse reactions in Europe and New Zealand
(Friis and Andreasen, 1992; Pillans, 1996). Similarly, liver injury secondary
to antitubercular drugs such as isoniazid continues to be reported worldwide
(Acharya et al., 1996; Lucena et al., 2001, Ostapowicz et al., 2002). As the relatively ‘high-risk’ agents have been replaced,
relatively rare reactions to commonly prescribed ‘low-risk’ agents have become
the most important cause of hepato-toxicity. Since 1995, non-steroidal
anti-inflammatory drugs (NSAIDs) such as diclofenac and sulindac; antimicrobials
such as co-amoxiclav, flucloxacillin and erythromycin; and H2 antagonists have
become important causes of hepatotoxicity (Pillans, 1996; Lucena et al., 2001). In addition,
hepatotoxicity due to substances that were previously thought to have little
toxicity, such as ‘Ecstasy’ (recreational amphetamine) and herbal remedies are
being increasingly recognised (Larrey, 1997; Andreu et al., 1998; Aithal, 2005). A brief list of the drugs, which are
important causes of hepatotoxicity and the pattern of the liver injury, is
shown in Table 35.1.

DIAGNOSIS OF ADVERSE HEPATIC REACTION
The
importance of drugs as a cause of liver injury lies not in the overall number
of cases but in the sever-ity of some reactions and their potential
reversibility, provided the drug aetiology is promptly recognised. Adverse
hepatic reactions can mimic a wide spec-trum of hepatobiliary diseases. Early
recognition and prompt withdrawal of the drug is essential in prevent-ing
serious hepatic failure and is the critical step in the management of adverse
reactions (Nolan, Goldberg and Buskin, 1999). Failure to detect hepatotoxicity
at an early stage has led to mortality in many reported cases of hepatotoxicity
(Moulding, 1999). The long-term prognosis of drug-induced hepatotoxicity may be
worse if the responsible agent is continued (Aithal and Day, 1999).
CAUSALITY ASSESSMENT METHODS
The
lack of specific tests for diagnosing drug hepa-totoxicity poses particular
problems for definitively attributing a liver reaction to an implicated drug.
The approach to the diagnosis of a drug-induced liver disease involves
physician awareness, the exclusion of other causes of the reaction and an
objective weigh-ing of the circumstantial evidence. The considera-tions have
been termed ‘causality assessment’ and form the cornerstone to the diagnosis of
drug-induced hepatotoxicity.
Decision
Tree Model
An algorithm-based model developed by Stricher (1992) considers three factors:
• the specificity of the clinico-pathological
pattern and its course,
• the temporal relationship between intake/
discontinuation of the suspected drug and onset/disappearance of hepatic injury
and
• the exclusion of other possible causes for
the observed pattern.
The
model assesses the degree of certainty of a causal relationship between hepatic
injury and drug intake; however, it has several major disadvantages. First, all
the factors are given equal weight; second, the quantitative data are reduced to
qualitative ‘yes’ or ‘no’ answers. Finally, categories such as ‘proba-ble’ and
‘possible’ lead to a semantic cause of inter-observer variation.
Bayesian Model
A
logical approach to the problem of causality assess-ment is based on Baye’s
theorem. This model uses the background incidence of an event, the individual
clin-ical features of a particular case and the probability of other potential
causes. The model estimates the prob-ability of a specific reaction in a
particular individual in a given situation being related to the drug therapy.
However, the Bayesian model is time-consuming and, hence, impracticable to use
in the evaluation of a large number of adverse hepatotoxic reactions. In
addition, the background incidence of a given reaction may not be known, thus
further limiting its use. In a large survey, the Bayesian model had an accuracy
of 62% in the diagnosis of drug-induced liver disease when compared with the
final diagnosis after investigations (Lavelle and Kavanagh, 1995).
International Consensus Criteria
In
1990, under the auspices of the Council for Interna-tional Organizations of
Medical Sciences (CIOMS), an international group of ‘experts’, proposed
defini-tions of adverse hepatotoxic reactions and criteria for assessing
causality of drug-induced liver disease to standardise the evaluation of drug
hepatotoxicity by physicians, health authorities of different countries and
pharmaceutical manufacturers (Benichou, 1990). For causality assessment, the
French method of ADR assessment was adapted to suit the evaluation of
drug-induced liver disease (Danan, 1988). ‘International consensus criteria’
combined the basic principles of ‘chronological criteria’ (establishing a
temporal rela-tionship between the drug treatment and reaction) and ‘clinical
criteria’ (the exclusion of alternative causes for the particular pattern of
liver injury) to deter-mine the probability of the reaction being related to
the drug. A detailed scoring system was devel-oped (CIOMS scale) and validated
using cases of drug-induced liver injury with known positive rechal-lenge
(Danan and Benichou, 1993). The CIOMS scale performed well when these cases
were assessed using the data prior to rechallenge. In a recent study (Aithal,
Rawlins and Day, 1999), 86% of the suspected hepatic ADRs could be classified
as ‘drug related’ or ‘unre-lated’ using a simplified form of consensus
classifi-cation (Table 35.2).

Even
though the ‘International consensus criteria’ are considered to be the ‘state
of art’, they cannot be used rigidly in all circumstances, especially to
exclude a drug as a cause of a given reaction. For example, the classification
of a causal relationship between a drug and cholestatic injury as ‘incompatible’
if the onset occurs more than a month after the last drug intake would unduly
refute such cases attributable to co-amoxiclav intake (Larrey et al., 1992a). Similarly,
flucloxacillin-induced cholestasis, which in one-third of patients may take up
to 18 months after the drug withdrawal to resolve (Turner et al., 1989), may be classified as ‘inconclusive’ according to the
consen-sus criteria. The CIOMS scale also defines alcohol, pregnancy and age
over 55 years as risk factors, which would reduce the flexibility to weigh
other risk factors relevant to the clinical setting.
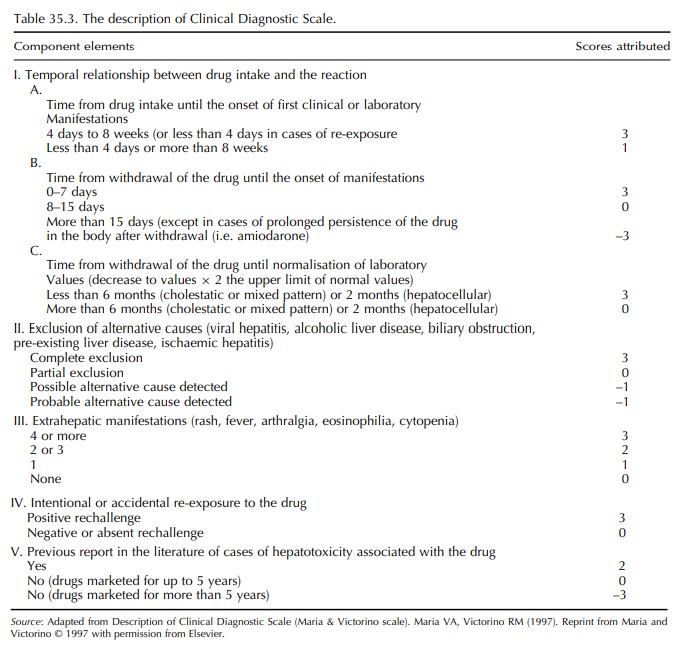
Clinical Diagnostic Scale
More
recently, a simplified scoring system called the ‘Clinical Diagnostic Scale’
(CDS) (otherwise called the Maria & Victorino scale) has been developed
(Maria and Victorino, 1997). Scores are attributed in seven different
components of a given reaction (Table 35.3), and the reactions are graded
according to the final score. The original validation of CDS used real and
fictitious cases and the opinion of the panel of experts as the gold standard.
A detailed comparison of the CIOMS scale and the CDS concluded that the latter
performed poorly while evaluating reactions with long latency periods and
evolution to chronicity after withdrawal (e.g. cholestasis due to amoxiclav)
(Lucena et al., 2001).
The CDS generally underscores the reactions. Even in the initial study, only four (all of which had positive rechallenge) were classified as defi-nite adverse hepatic reaction (score >17) (Maria and Victorino, 1997). The reason for low scoring is because of the emphasis given to positive rechallenge as well as extrahepatic manifestations (maximum of 3 scores each). Deliberate rechallenge of an incrim-inated drug is ethically unjustifiable, and inadver-tent re-exposure is reported in a minority (8.8%) of hepatic ADRs (unpublished data). Extrahepatic mani-festations, considered to represent immuno-allergic reaction, are infrequent with hepatotoxicity due to many of the currently used drugs (Banks et al., 1995; Hautekeete et al., 1999). None of the 180 patients in a large series of diclofenac hepatotoxicity would have scored maximum points for this component on the CDS (Banks et al., 1995). Even though underscoring by the CDS attributes to a lower level of probability to an individual drug-related hepatotoxic reaction, a cut-off CDS score of >9 still remains useful in group-ing the reactions that require further investigations and those wherein withdrawal of the drug is justified (Aithal, Rawlins and Day, 2000). Moreover, a numer-ical ‘cut-off’ is far easier to apply in routine clinical practice.
Systemic
evaluation using causality assessment methods such as international consensus
criteria or a CDS provides objectivity and consistency to the assessment of
suspected adverse hepatic drug reactions. Their more widespread adoption should
enhance the accuracy of case definition for epidemiological studies.
RECHALLENGE
The
recurrence of liver injury after re-administration (often inadvertent) of a
suspected drug is the most definitive evidence for drug-induced liver disease
and may outweigh other considerations in causality assess-ment. The biochemical
criteria for a positive rechal-lenge have been outlined by the consensus group
(Benichou, 1990). But, rechallenge of an incrimi-nated drug can be dangerous
and may even be fatal (Ransohoff and Jacobs, 1981; Lo et al., 1998). Delib-erate rechallenge may only be justified when
contin-ued treatment with the implicated agent is highly desirable.
ROLE OF LIVER BIOPSY
Drug-induced liver injury can cause any known pattern of liver pathology, although certain histological features are particularly suggestive of drug-induced aetiology (Anon., 1974). Liver biopsy is also an important way to exclude alternative causes of a given pattern of liver injury. However, liver biopsy is an invasive procedure with significant morbidity in 0.24% and mortality in 0.11% of subjects (Cohen et al., 1992). Hence, benefits should be weighed against the risk, and liver biopsy should be consid-ered only in circumstances where discontinuation of the suspected medication is undesirable or when a patient appears to have an as yet unrecognised form of drug-induced liver injury.
SPECIFIC TESTS
The
exceptions to the lack of ‘specific’ markers of drug hepatotoxicity are the
detection of liver–kidney microsomal type 2 (anti-P450 2C9) antibodies in
tienilic acid–induced hepatitis, antimitochondrial type 6 antibody in
iproniazid-induced hepatitis (Homberg et
al., 1985) and liver microsomal antibody (anti-P450 1A2) in dihydralazine-related liver injury (Bourdi et al., 1990). Both tienilic acid and
iproniazid were withdrawn because of
the high incidence of hepa-totoxicity, and dihydralazine is rarely used now in
clinical practice.
IN VITRO TESTS
The
difficulties encountered in the diagnosis of drug-induced liver injury have led
to attempts to develop in vitro
diagnostic tests. Assays have been devised to study the cytotoxic effect of
metabolites generated by a hepatic microsomal drug-metabolising system of the
peripheral blood mononuclear cells from patients suffering hepatotoxicity due
to pheny-toin and sulphonamides (Rieder et
al., 1989; Gennis et al., 1991).
The lymphocyte transformation test aims
to demonstrate in vitro proliferation
of a patient’s lymphocytes in response to the drug in question. Considering the
complexity of the immunological events necessary for the in vitro induction of specifi-cally sensitised T cells, it is not
surprising that the test is positive only in 30% of all patients with suspected
drug-induced liver injuries (Berg and Becker, 1995). The use of sera collected
from healthy volunteers after drug intake (containing ex vivo drug antigens) and the addition of prostaglandin inhibitors
to the cultures (to prevent the inhibition of lymphocyte response by
prostaglandin-producing suppresser cells) can increase the sensitivity of the
test up to 56% (Maria and Victorino, 1998). However, the fact that these in vitro tests are tedious and operator
dependent has limited their widespread use.
Related Topics
