Strategies for the Detection and Prevention of Idiosyncratic Haematological ADRS
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Gastrointestinal ADRs
Regular full blood count (FBC) monitoring is clearly indicated when drugs associated with type A haematological ADR, such as cytotoxic agents, are prescribed.
STRATEGIES FOR THE DETECTION AND
PREVENTION OF IDIOSYNCRATIC HAEMATOLOGICAL ADRS
INDIVIDUAL MONITORING
Regular
full blood count (FBC) monitoring is clearly indicated when drugs associated
with type A haematological ADR, such as cytotoxic agents, are prescribed. For
idiosyncratic reactions, early warning rather than prevention is the main goal.
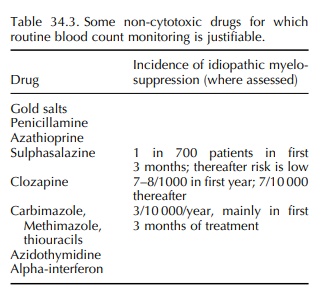
For a small number of drugs with a significant risk of myelosup-pression,
regular monitoring, as for cytotoxic therapy, is required or desirable (Table
34.3). Patient and carer education in the significance of symptoms sugges-tive
of infection, bleeding and anaemia are again important. Monitoring may prevent
a minor cytope-nia developing into a more severe aplasia by indi-cating the
discontinuation of gold or penicillamine therapy where a prodromal gradual
count reduction may precede a severe reaction. Monitoring itself will clearly
not prevent a suddenly precipitate agranulocy-tosis with, e.g., antithyroid
drugs, which may occur in between even quite frequent monitoring visits. It
does however reinforce patient education in the potential complication, and
their access to FBC increasing the likelihood of early detection.
The
case for routine surveillance monitoring with antithyroid drugs is
controversial (Drug and Therapeutics Bulletin, 1997a,b). A prospective study in
Japan found a 0.4% incidence of agranulocyto-sis occurring within the first 3
months of treatment with methimazole or propylthiouracil, and 43 of 55 the
affected patients were detected by routine moni-toring before the onset of
symptoms (Tajiri et al., 1990).
Counts recovered in all the patients, and 29 did not develop any infection.
Monitoring clearly allowed the prevention of a potentially dangerous
complication for a significant group of patients in this study, but the
pharmacoeconomic justification for routine monitoring in this situation is not
universally accepted.
INDIVIDUAL RISK-FACTOR IDENTIFICATION
In
addition to FBC monitoring, pre-treatment assess-ment of TPMT either by enzyme
activity or by genetic markers before azathioprine or 6-MP treatment and MTHFR
status before MTX therapy, as discussed above, may assist prevention. It is
likely that addi-tional predictive tests will become applicable as
phar-macogenetic knowledge increases.
SPONTANEOUS REPORTING
The
notification of suspected occurrences of drug-induced myelosuppression to
national licensing authorities is an important contribution to prevention, and
particularly important for idiosyncratic reactions to new agents. The UK
‘Yellow Card’ scheme informs an ADRs On-line Information Tracking (ADROIT)
database that captures all reports for separate drugs and categorises
haematological reactions into non-serious, serious and fatal categories. Whilst
such data, which have no reliable numerator, cannot define inci-dences of
reactions, they can highlight suspicions of new potentially significant
reactions and follow trends in frequency and severity of established reactions.
Related Topics
