Inhalation Products
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Solid Dosage Forms
Solid particles are employed in two types of inhalation product: the pressurized metered-dose inhaler (pMDI) and the dry powder inhaler (DPI).
INHALATION PRODUCTS
Solid
particles are employed in two types of inhalation product: the pressurized
metered-dose inhaler (pMDI) and the dry powder inhaler (DPI). In both cases,
the method of choice for manufacturing particles in an appropriate size range
to deposit in the lungs (<5
μm) is attrition
milling by air jet mill.
The
pMDI product is prepared as a nonaqueous suspension in which surfactant is used
to disperse the drug particles in high–vapor pressure pro-pellants. Once the
particles are prepared, the product formulation depends only on the particle
dispersion in suspension, their ease of redispersion, and their physical
stability upon aerosolization. Figure 14.9 shows a typical filling line for
pMDI product.
DPI
formulations usually involve a combination of the micronized drug with a
carrier, notably lactose. The carrier particles are usually larger than the
drug particles and outside the range required for lung deposition (>30 mm). The purpose of these large
particles is to help disperse the respirable drug particles carrying them into
the inspiratory airflow where they are stripped from the surface as a function
of the large shear forces. These formulations are prepared in capsules,
blisters, or reservoir devices. The filling technology has been developed to
accurately meter small doses into the unit-dose packaging.
Other
methods of particle preparation have been evaluated, including spray drying and
supercritical fluid manufacture. The capacity to manufacture particles with
known and optimized particle size, shape, and surface charac-teristics is
intriguing, and it seems likely that these methods will become more significant
in the future and may even surpass micronization for aerosol delivery of drugs.
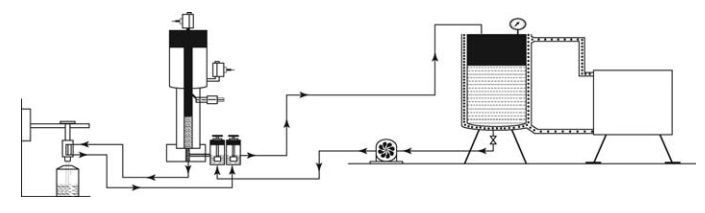
FIGURE 14.9 Metered-dose inhaler filling line.
