Tablets
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Solid Dosage Forms
Additional processes are required for tablet production beyond those described previously.
TABLETS
Additional
processes are required for tablet production beyond those described previously.
Because these processes are not ubiquitous in pharmaceutical manufacturing,
they are dealt with only briefly here. Many of them are required for all solid
dosage forms. Each process must be conducted while balancing the effects of the
respective excesses.
Compressed
solids, tablets, or caplets are prepared by placing the blend of component
additives in a cylinder or die, above a movable piston or punch. An upper punch
is brought into the top of the piston, and pressure applied to the distal ends
of the punches forces the powder into a compact (Fig. 14.7). Product quality
depends on the cohesive forces acting on the powder under compres-sion. These
cohesive forces are influenced by the selection of additives in the dosage
formulation. One method of evaluating tablet manufacture considers the effect
of applied pressure on porosity of the compressed powder (Carstensen, 1993).
Data may be plotted as the negative natural logarithm of porosity against
applied pressure in the form of a Heckel plot (Heckel, 1961). The slope is
pro-portional to the yield value (φ, elastic limit) l/3φ.
The
tooling of a tablet press varies according to the tablet design. Con-sideration
must be given to the distribution of forces across the faces of the tablet
punches as they are brought together to compress the tablet in the die. As more
unusually shaped tablets are produced and more elaborate embossing tools are
required, the forces are not distributed evenly across the punches, and care
must be taken if they are to have a reasonable, useful life span.
Mathematically, finite element analysis can be used to characterize these
forces and to calculate the requirement for preserving the tools· for extended
periods.
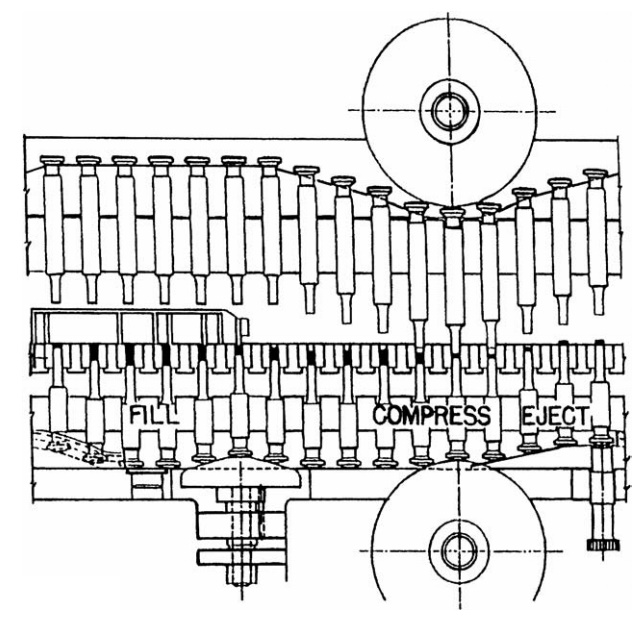
FIGURE 14.7 Tablet manufacture depicting the
three steps of filling, compression, and ejection.
Coating is achieved
by placing a batch of tablets in a coating pan and spraying or coating from
solution with the required polymer. The Accela-Cota (Fig. 14.8) is one of the
more common coating systems.
Tablets have been
prepared with different characteristics and for different purposes. The most
common tablets are uncoated, coated, chewable, or effer-vescent. Some
specialized dosage forms have been developed for sublingual and buccal
delivery. A typical uncoated conventional tablet might have the com-position
shown in Table 14.1. Examples of such systems include generic aspirin and
Valium. These tablets are designed for rapid dissolution.
Tablets may be
coated for a variety of reasons, including better appear-ance, taste masking,
ease of swallowing, protection from light, protection from gastrointestinal
irritation, facilitating tablet printing, and control release. The formulation
of a coated tablet is similar to that of an uncoated tablet. Usually, it is
coated from a solution of polymer, for example, methylcellulose, enteric
polymer. Bayer aspirin or erythromycin products are examples of coated tablets.
Chewable
tablets are usually flavored and contain additives that contrib-ute to a smooth
texture, including glycerin and sugars such as mannitol and sorbitol. An
example is Tylenol chewable tablets.
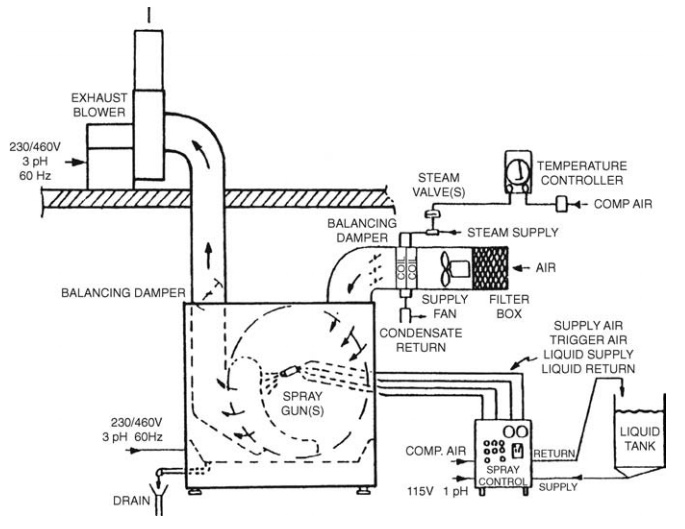
FIGURE 14.8 Accela-Cota. Source: Courtesy of Thomas Engineering, Hoffmann Estates,
Illi-nois, U.S.
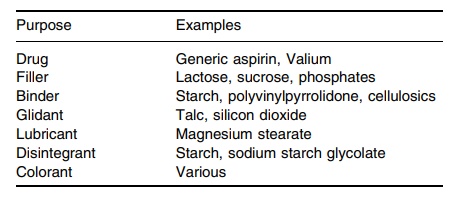
TABLE 14.1 Tablet Composition
Effervescent tablets
are formulated so that an acid-base reaction occurs when they are combined with
water. This is achieved by using weak acids (e.g., citric, malic, tartaric, or
fumeric acids) or bases (e.g., sodium or potassium carbonates) in the product.
The best known of these products is Alka-Seltzer.
Sublingual
tablets are designed to disintegrate and dissolve instantly. Hence, they must
have structural integrity sufficient for storage, transport, and administration
but capable of dissolution on the oral mucosa under the tongue. Nitroglycerin
tablets designed for treating angina are prepared in a composi-tionally simple
formulation of lactose massed with 60% ethanol. This route of administration is
intended to avoid first-pass liver metabolism. Testosterone tablets have been
prepared for buccal delivery by slow dissolution. The tablet does not contain a
disintegrant and is intended to have an extended residence time in the buccal
cavity at the rear of the mouth. Since release is not immediate, drug dosage
may be significantly reduced by this route.
