Magnesium: competition to lithium?
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Alkaline Earth Metals
The element magnesium (Mg) is a silvery-white and lightweight metal. It is protected by a thin oxide layer, which is very difficult to remove but at the same time removes the need to store it in an oxygen-free environment (see alkali metals).
Magnesium:
competition to lithium?
The element magnesium (Mg) is a silvery-white and lightweight
metal. It is protected by a thin oxide layer, which is very difficult to remove
but at the same time removes the need to store it in an oxygen-free
environment (see alkali metals). Magnesium reacts with water but much more
slowly than its neighbouring earth alkaline metal calcium. Magnesium is a
highly flammable metal, and once ignited it burns with a characteris-tic bright
white flame. There are three stable isotopes of magnesium, namely 24Mg
(79% occurrence), 25Mg and 26Mg. 28Mg is
radioactive with a half-life of 21 h .
Most magnesium salts are soluble in water, and given in large
amounts they work as a laxative in the human body. Aqueous magnesium ions are
sour in taste. Magnesium hydroxide (MgOH2) has only limited
solubility in water and the resulting suspension is called milk of magnesia, which is commonly used as an antacid and is known
to be a mild base. Magnesium is extracted on a large scale using a Down’s
cell or the ferrosilicon process with
seawater being the main source. Mg2+ stands in a so-called diagonal
relationship to Li+, which explains why these ions have similar
properties and biological activity.
Biological importance
Mg2+ is an essential ion in the human body and is a
crucial constituent in numerous enzymatic processes. Indeed, Mg2+ is
essential to most living cells as a signalling molecule and is involved in
nucleic acid bio-chemistry dealing with the manipulation of ATP (adenosine
triphosphate), DNA, RNA and related processes. For example, ATP has to be
coordinated to a magnesium ion in order to become biologically active. Mg2+
also stabilises DNA and RNA structures, which can be seen in their increased
melting points.
Mg2+ ions form the redox-active centre in
chlorophyll, which facilitates the process of photosynthesis and the connected
carbon fixation in green plants. Therefore, green vegetables, as well as milk,
whole grain and nuts, are good sources of magnesium. It has to be kept in mind
that most magnesium salts are water soluble and therefore processed vegetables,
mainly cooked in water, are low in magnesium ion content.
In the human body, Mg2+ is the fourth
most abundant cation and the second most abundant ion in the interstitial
fluid. Mg2+ is an essential co-factor dealing with more than 300
cellular enzymatic processes.
On average, the human body contains about 24 g of magnesium
ions, with half of it being incorporated into bones and the other half being
present in muscles and soft tissue. The majority of Mg2+ is absorbed
in the ilium and colon, and the kidneys are the major excretory organ. Mg2+
is filtered at the glomerulus, and 10–15% is re-absorbed at the proximal
tubule, 60–70% at the thick part of the ascending limb of the loop of Henle and
10–15% at the distal tubule .
Nevertheless, magnesium salts are generally not well absorbed
from the gastrointestinal (GI) tract and therefore are often used as osmotic
laxatives. The kidneys regulate the magnesium ion levels in plasma, and as a
result high levels of Mg2+ are retained when the patient has renal
failure. The resulting hypermagnesia can cause muscle weakness and arrhythmia,
but it is a rare condition. Hypomagnesia, defined as low magne-sium levels in
the blood plasma, can be the result of losses in the GI tract, for example,
excessive diarrhoea. Magnesium imbalances can also be a result of alcoholism or
secondary to treatment with certain drugs. Hypo-magnesia is often followed by
hypocalcaemia (low calcium ion plasma levels) as well as hypokalaemia and
hyponatraemia.
Clinical applications and preparations
Magnesium ion imbalances can manifest in a variety of conditions
such as hypo- and hypermagnesaemia. Magnesium ion preparations are also used as
antacids, mostly in combination with aluminium-based salts. Additionally,
magnesium salts are involved in the treatment of arrhythmia (irregular heart
beat) and eclampsia, a life-threatening hypertensive disorder in pregnant
women.
Symptomatic hypomagnesaemia is associated with plasma serum Mg2+
levels of <0.5–1
mmol/kg for a period of 5 days or more. Mg2+ ions are initially
given as intravenous (i.v.) or intramuscular injection; the latter is fairly
painful and consisting of magnesium sulfate (MgSO4). MgSO4
can also be used as emergency treatment for very serious arrhythmias, a
disorder of the heart rate (pulse). In an emergency treatment, it is usually
given intravenously as one single dose or with one repeat (Figure 3.5) .
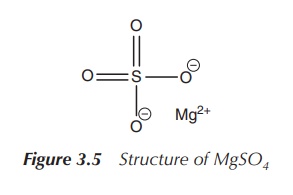
Note that the plasma magnesium concentration should be
monitored, and the dose has to be reduced in patients with renal impairment as
Mg2+ is excreted via the kidneys. Magnesium ions can also be given
orally to the patient, for example, in the form of magnesium glycerophosphate
tablets.
Magnesium hydroxide [Mg(OH)2] is present in antacids
because of its laxative properties and is also the main ingredient of the ‘milk
of magnesia’. The ‘milk of magnesia’ is a suspension of Mg(OH)2 in
water, which has a milk-like appearance because of the low aqueous solubility
of Mg(OH)2. It is considered as a strong electrolyte and a weak base
and is given to the patient for indigestion and heartburn. The alkaline
suspension neutralises any excess stomach acid and therefore works as an
antacid. It also stimulates intestinal movement, as the magnesium ions
increases the water content in the intestines through its osmotic effect and as
a result softens any faeces present.
Magnesium trisilicate (Mg2Si3O8)
can also be used in antacid preparations especially in the treatment of peptic
ulcers. The mode of action includes the increase of the pH of the gastric fluid
together with the formation of a colloidal silica precipitate, which forms a
protection for the GI mucosa. Most antacids contain a mixture of
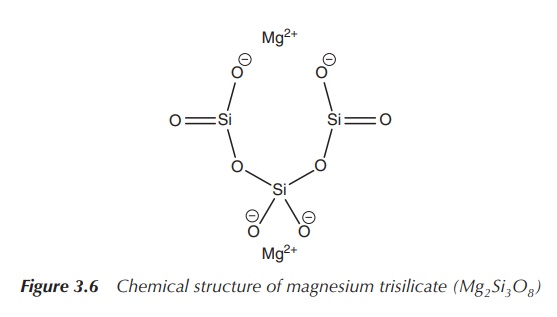
Unfortunately, orally taken magnesium salts can show
interactions with other drugs taken simultaneously. Magnesium trisilicate
reduces the absorption of iron products, certain antibiotics (such as
Nitrofurantoin) or antimalarial drugs (such as Proguanil). Magnesium salt
preparations, which form part of antacids, are not recommended to be taken at
the same time as a variety of drugs such as ACE inhibitors, aspirin and
peni-cillamine. In most cases, antacids reduce the absorption of the simultaneously
taken drug. Therefore, before any treatment with antacids, the full medical
history of the patient should be taken and possible interactions assessed .
Related Topics
