Calcium: the key to many human functions
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Alkaline Earth Metals
Calcium is the most abundant inorganic element in the human body and is an essential key for many physio-logical processes. Ca2+ has numerous intra and extracellular physiological roles, for example, a universal role as messenger and mediator for cardiac, skeletal and smooth muscle contractions.
Calcium:
the key to many human functions
Calcium is the most abundant inorganic element in the human body
and is an essential key for many physio-logical processes. Ca2+ has
numerous intra and extracellular physiological roles, for example, a universal
role as messenger and mediator for cardiac, skeletal and smooth muscle contractions.
Calcium ions are a critical factor in several life-defining biochemical
processes as well as in the endocrine, neural and renal aspects of blood
pressure homeostasis.
Calcium has the symbol Ca and atomic number 20
and is a soft grey alkaline earth metal. Calcium has four stable isotopes (40Ca
and 42Ca–44Ca) and the metal reacts with water with the
formation of calcium hydroxide and hydrogen.
2Ca + 2H2O → 2CaOH
+ H2 (3.3)
Calcium salts can be found in everyday life. Limestone, cement,
lime scale and fossils are only a few examples where we encounter Ca2+.
They also have a wide spectrum of applications spanning from insecticides to clinical
applications. Calcium arsenate [Ca3(AsO4)2] is
extremely poisonous and is used in insecticides. Calcium carbonate (CaCO3)
can be found in clinical applications such as antacids, but note that an
excessive intake can be hazardous. Calcium chloride (CaCl2) is used
in ice removal and dust control on dirt roads, as a conditioner for concrete
and as an additive in canned tomatoes. Calcium cyclamate [Ca(C6H11NHSO4)2]
is used as a sweetening agent, and calcium gluconate [Ca(C6H11O7)2]
is used as a food additive in vitamin pills. Calcium hypochlorite Ca(OCl)2
can be found in swimming pool disinfectants, in bleaching agents, in deodorants
and in fungicides. Calcium permanganate [Ca(MnO4)2] is
used in textile production, as a water-sterilising agent and in dental
procedures. Calcium phosphate [Ca3(PO4)2]
finds applications as a supplement for animal feed, as a fertiliser, in the
manufacture of glass and in dental products. Calcium sulfate (CaSO4⋅2H2O) is the common blackboard
chalk.
Biological importance
Calcium ions play important roles in the human body in a variety
of neurological and endocrinological processes. Calcium is known as a cellular messenger and it has a large
intra- versus extracellular gradi-ent (1 : 10 000), which is highly regulated
by hormones. This gradient is necessary to maintain the cellular responsiveness
to diverse extracellular stimuli. Calcium ions are also involved in the
formation of bones and teeth, which act also as a reservoir for calcium ions.
A normal adult body contains ∼1000 g of calcium, of which around 99% are extracellular and
most of which is stored in bones and teeth. Bones actually serve as a dynamic
store for Ca2+. The remaining 1% of Ca2+ can be found in
the extracellular space, such as plasma, lymph and extracellular water. The
intra and extracellular Ca2+ concentration is extremely important to
many physiological functions and is therefore rigorously controlled (Figure
3.7) .
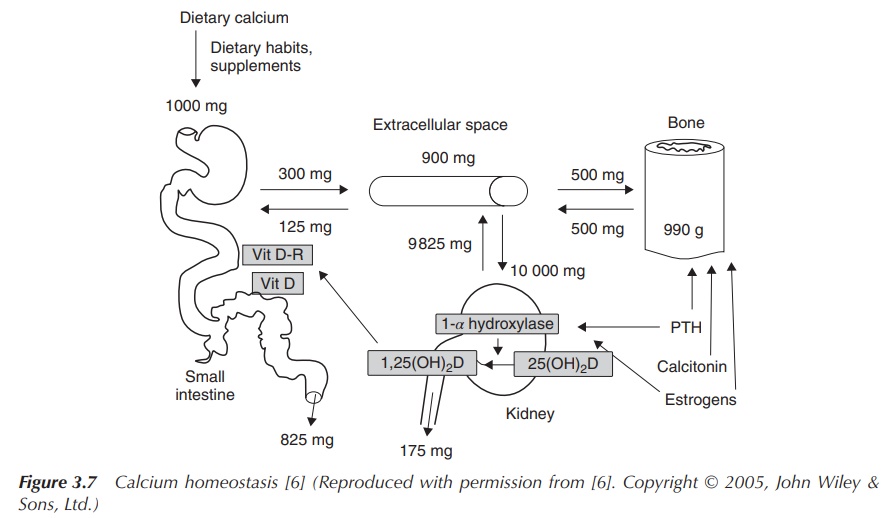
Calcium ions are regulated within the gut, skeleton and kidneys.
The Ca2+ homeostasis is normally in equi-librium, which means that
the amount of Ca2+ enters the body is equal to the amount of Ca2+
leaving the body. Calcium ion levels are regulated by hormones that are not
regulated by the Ca2+ level, called noncalciotropic hormones,
for example, sex hormones and growth factors. In contrast, there are hormones
that are directly related to Ca2+,
for example, PTH (parathyroid hormone), which are called calciotropic hormones. PTH con-trols the serum plasma level of Ca2+
by regulating the re-absorption of Ca2+ in the nephron, stimulating
the uptake of Ca2+ from the gut and releasing Ca2+ from
the bones which act as a reservoir.
Modified hydroxylapatite, also frequently called hydroxyapatite and better known as bone mineral, makes up ∼50% of our bones. Hydroxylapatite is a natural form of the mineral calcium apatite, whose formula is usually denoted as Ca10(PO4)6(OH)2. Modifications of hydroxylapatite can also be found in the teeth, and a chemically identical substance is often used as filler for replacement of bones, and so on. Nevertheless, despite similar or identical chemical compositions, the response of the body to these compounds can be quite different.
How does dietary calcium intake influence our lives?
It is believed that an optimal dietary calcium intake can
prevent chronic diseases. In the Stone Age, the average calcium intake was
2000–3000 mg Ca2+/day per adult, whereas now-a-days it has decreased
to an average of 600 mg/day . This means that we are living in permanent
calcium deficiency, and it is believed that there are linkages to various
chronic diseases, such as bone fragility, high blood pressure and colon cancer
.
Ca2+ is an essential nutrient, and the required
amount varies throughout a person’s life time depending on the stage of life.
There have been three stages of life identified when the human body needs an
increased level of Ca2+. The first one is childhood and adolescence
because from birth to the age of ∼18 the bones form and grow until they reach their maximum
strength. Pregnancy and lactation has also been identified as a time when the
human body is in need of an increased level of Ca2+. A full infant
accumulates around 30 g of Ca2+ during gestation and another 160–300
mg/day during lactation. Ageing has been identified as the third period of life
in humans when increased calcium intake is required. This has been associated
with several changes to the calcium metabolism in the elderly (Table 3.2).
Calcium deficiency: osteoporosis, hypertension and weight management
Osteoporosis is most commonly associated with calcium deficiency, but an adequate calcium intake should not only be considered as a therapy for bone loss. It should be seen as an essential strategy for the maintenance of health in the ageing human. Ninety-nine percent of Ca2+ is found in the bones, as they function as a reservoir.
Osteoporosis is known
to be the major underlying cause on bone fractures in postmenopausal women.
Calcium uptake and plasma concentrations are closely regulated by hormones, as
outlined in previous Section . Nevertheless, there has been no clear and direct
relationship between Ca2+ intake and bone health established until
now. It is believed that a high Ca2+ concentration and vitamin D
level is essential in the first three decades of life in order to establish an
optimum bone density level. These also modify the rate of bone loss, which is
associated with ageing.
Studies support the hypothesis that calcium supplementation can
reduce blood pressure, being more ben-eficial to salt-dependent hypertension.
The regulation of the cellular calcium metabolism is central to blood pressure
homeostasis. It is believed that the higher the level of cytosolic-free calcium
ions, the greater the smooth muscle vasoconstrictor tone, which in turn has an
effect on the sympathetic nervous system activity and thus on the blood
pressure. Nevertheless, studies do not justify the use of calcium
supplementation as the sole treatment for patients with mild hypertension.
It has been hypothesised that there exists a link between
dietary calcium and weight management in humans. It has been proposed that a
low-calorie, high-Ca2+ diet helps in supporting the fight against obesity
and increase the energy metabolism. The recommended Ca2+ intake
should be around 1200 mg/day as previously mentioned depending on the age.
Available evidence indicates that increasing the calcium intake may
sub-stantially reduce the risk of being overweight, although long-term,
large-scale prospective clinical trials need to be conducted to confirm or
better clarify this association.
Renal osteodystrophy
Renal osteodystrophy, also called renal bone disease, is a bone mineralisation deficiency seen in
patients with chronic or end-stage renal failure.
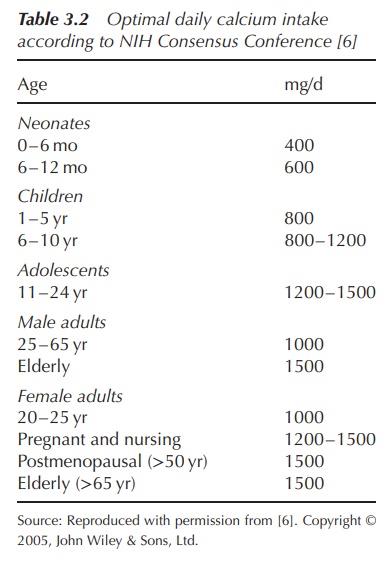
Vitamin D is usually activated in the liver to the pro-hormone
calcidiol and then in the kidney to calcitriol, which is the active form of
vitamin D. Both activation steps are based on a hydroxylation reaction.
Pro-vitamin D is hydroxylated in the 25 position in the liver (calcidiol) and
then in the kidney at the 1α-position (calcitriol). Calcitriol helps the body
to absorb dietary Ca2+ (Figure 3.8).
In patients with renal failure, the activation to calcitriol is depressed, which results in a decreased con-centration of Ca2+ in the blood plasma. Furthermore, the plasma phosphate level increases as a result of the kidney impairment. This, in turn, reduces the amount of free Ca2+ in the blood even more, as the phosphate complexes the free Ca2+. The pituitary gland senses the low levels of plasma Ca2+ and releases PTH. As previously outlined, PTH increases the re-absorption of Ca2+ in the nephron and absorption in the gut, and promotes the release of Ca2+ from the bones. In turn, this leads to a weakening of the bone structure.
Patients can be treated with phosphate binders in order to avoid
excess phosphate absorption from the gut. Dialysis will also be helpful in
removing excess phosphate from the blood. Furthermore, the patient can be given
synthetic calcitriol and potentially calcium supplements.
Kidney stones
Around 20–40% of all kidney stones are associated with elevated
Ca2+ level in the urine. For a long time, it has been suggested that
low dietary calcium intake would be the best method to prevent the recurrence
of kidney stones. More recent studies involving patients who suffered from
recurring calcium oxalate stones showed that a low calcium diet did not prevent
the formation of kidney stones. It was actually found that a higher calcium
intake of around 1200 mg/day resulted in a significant reduction of the
recurrence of kid-ney stones by around 50%. It is believed that the restriction
of calcium leads to an increase in absorption and excretion of oxalate in the
urine and therefore promotes the formation of calcium oxalate stones. Cur-rently,
the conclusion is that kidney stone formation in healthy individuals is not
associated with calcium supplementation .
Clinical application
Calcium supplements are usually required only if the dietary Ca2+
intake is insufficient. As previously men-tioned, the dietary requirements
depend on the age and circumstances; for example, an increased need can be seen
in children, in pregnant women and in the elderly where absorption is impaired.
In severe acute hypocalcaemia, a slow i.v. injection of a 10% calcium gluconate
has been recommended. It has to be kept in mind that the plasma Ca2+
level and any changes to the electrocardiogram (ECG) have to be carefully
monitored .
A variety of calcium salts are used for clinical application,
including calcium carbonate, calcium chloride, calcium phosphate, calcium
lactate, calcium aspartate and calcium gluconate. Calcium carbonate is the most
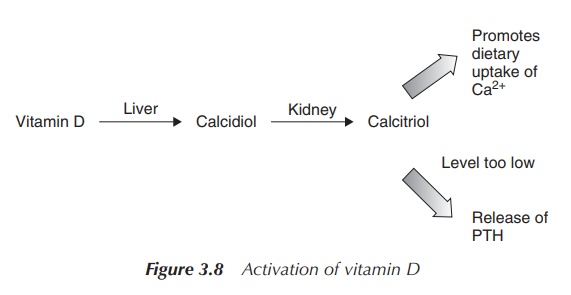
common and least expensive calcium supplement. It can be difficult to digest
and may cause gas in some people because of the reaction of stomach HCl with
the carbonate and the subsequent production of CO2 (Figure 3.9).
CaCO3 + 2HCI → CO2 + CaCl2 + H2O
Figure 3.9 Chemical equation showing the synthesis of CO2 under acidic stomach conditions
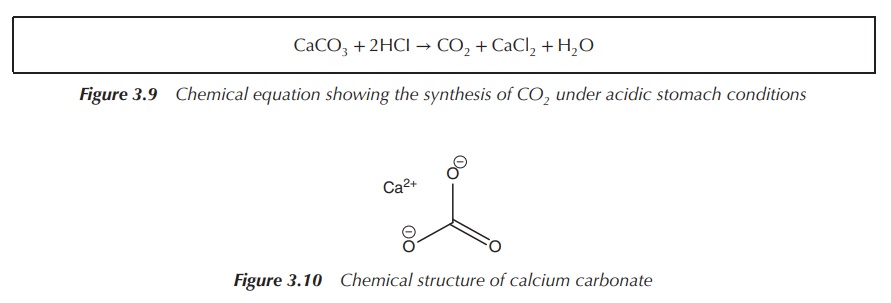
Calcium carbonate is recommended to be taken with food, and the absorption rate in the intestine depends on the pH levels. Taking magnesium salts with it can help prevent constipation. Calcium carbonate consists of 40% Ca2+, which means that 1000 mg of the salt contains around 400 mg of Ca2+. Often, labels will only indicate the amount of Ca2+ present in each tablet and not the amount of calcium carbonate (Figure 3.10).
Calcium citrate is more easily absorbed (bioavailability is 2.5
times higher than calcium carbonate); it is easier to digest and less likely to
cause constipation and gas than calcium carbonate. Calcium citrate can be taken
without food and is more easily absorbed than calcium carbonate on an empty
stomach. It is also believed that it contributes less to the formation of
kidney stones. Calcium citrate consists of around 24% Ca2+, which
means that 1000 mg calcium citrate contains around 240 mg Ca2+. The
lower Ca2+ content together with the higher price makes it a more
expensive treatment option compared to calcium carbonate, but its slightly
different application field can justify this (Figure 3.11).
The properties of calcium lactate are similar to those of
calcium carbonate , but the former is usually more expensive. Calcium lactate
contains effectively less Ca2+ per gram salt than, for example,
calcium carbonate. Calcium lactate consists of only 18% Ca2+, making
it a less ‘concentrated’ salt (Figure 3.12) .
Calcium gluconate is prescribed as a calcium supplement, but it
is also used in the urgent treatment of hyperkalaemia (K+ plasma
levels above 6.5 mmol/l). Hyperkaleamia in the presence of ECG changes usually
requires immediate treatment, and a 10% calcium gluconate solution
intravenously administered is recommended. Administration of the calcium
solution does not lower the plasma K+ level but protects temporarily
against myocardial excitability and therefore temporarily reduces the toxic
effects of hyperkalaemia. Calcium gluconate contains effectively the least Ca2+
per amount of supplement (only around 9%). That means that in 1000 mg calcium
gluconate, only 90 mg is actual Ca2+ (Figure 3.13).
Side effects
Several large long-term studies have shown that a daily intake
of 1000–2500 mg of calcium salts is safe. Side effects have been observed only
at relatively high doses, being manifested in GI disturbances such as
constipation and bloating and, in extreme cases, arrhythmia . The GI system
normally adjusts after a while, and problems should resolve themselves. Calcium
salts are generally better absorbed in an acid environment, so patients with a
low production of stomach acid or elderly patients who are on high doses of
antiulcer medication might experience problems with absorption. It is then
recommended to consume the calcium supplement with a meal .
Nevertheless, it is important to note that calcium ions can
interfere with the absorption of some drugs, such as antibiotics. For example,
tetracycline and quinolone antibiotics can chelate Ca2+ ions and
form complexes which cannot be absorbed anymore. Therefore, calcium supplements
and antibiotics should not be taken together. Patients are typically advised to
take antibiotics 1 h before or 2 h after food .
Related Topics
