Earth alkaline metal ions
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Alkaline Earth Metals
Earth alkaline metals together with the alkali metals form the so-called s-block metals. Earth alkaline metals have two electrons in their outer shell which is an s-orbital type.
Earth
alkaline metal ions
Earth alkaline metals together with the alkali metals form the
so-called s-block metals. Earth alkaline metals have two electrons in their
outer shell which is an s-orbital type. The chemistry of the metals is
characterised by the loss of both electrons, which is a result of the
relatively low ionisation energy (IE) of both electrons and the subsequent
formation of the stable cation M2+, which has a noble gas
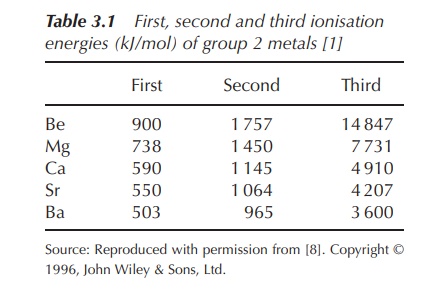
configuration (Table 3.1).
Group 2 elements are all silvery-white metals with high
reactivity, similar to alkali metals, but less soft and not as reactive. Earth
alkaline metals can be mostly found in the earth’s crust in the form of their
cations displayed in minerals and not as the elemental metal, as these are very
reactive. For example, beryllium principally occurs as beryl (Be3Al2[Si6O18]),
which is also known as aquamarine.
Magnesium can be found in rock structures such as magnesite
(MgCO3) and dolomite (MgCO3⋅CaCO3), and is the eighth most abundant element in
the earth’s crust. Calcium is the fifth most abundant element and can be found
in minerals such as limestone (CaCO3) and its metamorphs such as
chalk and marble.
Earth alkaline metals are harder and have a
higher density than sodium and potassium and higher melting points. This is
mostly due to the presence of two valence electrons and the resulting stronger
metallic bond. Atomic and ionic radii increase within the group, and the ionic
radii are significantly smaller than the atomic radii. Again, this is due to
the existence of two valence electrons, which are located in the s orbital
furthest from the nucleus. The remaining electrons are attracted even closer to
the nucleus as a result of the increased
The IEs of the first
two valence electrons are similar and relatively low compared to the energy
needed to remove the third valence electron, which is part of a fully filled
quantum shell. As a result, the dominant oxidation state of earth alkaline
metals is +2.
Major uses and extraction
Beryllium is one of the lightest metals and therefore is used in
high-speed aircrafts and missiles. Unfor-tunately, it is highly toxic, and CBD,
a scarring of the lung tissue, is often seen in workers from within a
beryllium-contaminated work environment.
Calcium is mostly found in limestone and its related forms, such
as chalk, and marble and lime (CaO). Ca2+ ions are essential for
living organisms, as is Mg2+. Magnesium is the only earth alkaline
metal that is used on an industrial scale. It is used in ammunition (e.g.
tracer bullets and incendiary bombs), as it burns with a very bright white
glow. Magnesium alloyed with aluminium results in a low-density and strong
material, which is used for lightweight vehicles and aeroplanes.
Magnesium is the only group 2 element that is extracted on a
large scale. Its main source is seawater, and the metal is extracted by adding
calcium hydroxide. Magnesium hydroxide precipitates, as it is less soluble in
water compared to the calcium compound. Magnesium hydroxide is converted into
magnesium chloride (MgCl2), which can be subsequently electrolysed
in a Down’s cell in order to produce the
pure magnesium metal (Figure 3.2).
Conversion 2HCl + Mg(OH)2 → MgCl2 +
2H2O
Electrolysis: at the cathode: Mg2+ + 2e–→
Mg
at the anode: 2Cl–(l) Cl2(g) + 2e–
Redox: 2Mg2+ + 2Cl–→ 2 Mg + Cl
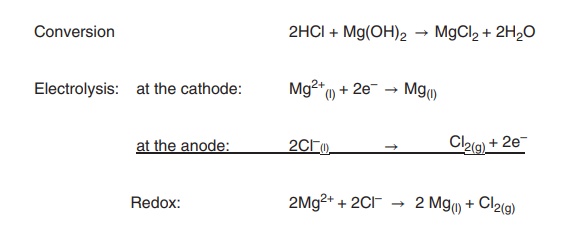
Figure 3.2 Redox equation for the production of magnesium
Calcination ∶ Dolomite [CaMg(CO3)2] is converted into
MgO and CaO
Reduction ∶ 2MgO + 2CaO + FeSi → 2Mg + Ca2SiO4
+ Fe + 1450 K
Figure 3.3 Chemical
equation for the production of magnesium
Alternatively, there is a second method called the ferrosilicon process or pigeon process. This involves the
reduction of magnesium oxide, which is obtained from dolomite, with an
iron–silicon alloy. The raw material has to be calcined first, which means the
removal of water and carbon dioxide, as these would form gaseous by-products
and would reverse the subsequent reduction (Figure 3.3).
Chemical properties
The chemical behaviour of alkaline earth metals is characterised
by their strong reducing power, and there-fore they very easily form bivalent
cations (M2+). The elements within group 2 become increasingly more
electropositive on descending within the group.
The metals themselves
are coloured from grey (Be, Mg) to silver (Ca, Sr, Ba) and are soft. Beryllium
and magnesium are passivated and therefore kinetically inert to oxygen or
water. The metal barium has to be stored under oil because of its reactivity.
Metals such as calcium, strontium and barium react similar to sodium, but are
slightly less reactive: All the metals except beryllium form oxides in air at
room temperature once the reaction is started. The nitride compound is formed
in the presence of nitrogen, and magnesium can burn in carbon dioxide, which
means that magnesium fires cannot be extinguished by the use of carbon dioxide
fire extinguishers.
2M+O2 →2MO 3M+N2 →M3N2
2Mg(s) + CO2(g) → 2MgO(s)
+ C(s)
The oxides of
alkaline earth metals have the general formula MO and are generally basic.
Beryllium oxide (BeO) is formed by the ignition of beryllium metal in an oxygen
atmosphere. The resulting solid is colourless and insoluble in water. Other
group 2 oxides (MO) are typically formed by the thermal decomposition of the
corresponding metal carbonate or hydroxide.
MCO3 → MO + CO2
Be(OH)2 + 2(OH)− → [Be(OH)4]2−
Be(OH)2 + H2SO4
→ BeSO4 + 2H2O
Figure 3.4 Chemical
equations showing the amphoteric nature of beryllium hydroxide
Peroxides
are known for magnesium, calcium, strontium and barium but not
for beryllium. The radius of the
beryllium cation (Be2+) is not sufficient to accommodate the peroxide
anion.
Beryllium reacts with aqueous alkali (NaOH)
and forms beryllium hydroxide, which
is an amphoteric hydroxide.
The
term amphoteric describes compounds
that can act as an acid and a base.
Beryllium hydroxide reacts with a base with the formation of the
corresponding beryllium salt. Beryllium hydroxide can also be reacted with
acids such as sulfuric acid and the corresponding salt, beryllium sulfate, is
obtained (Figure 3.4).
Magnesium does not react with aqueous alkali
(NaOH). The synthesis of magnesium hydroxide [Mg(OH)2] is based on a
metathesis reaction in which magnesium salts are reacted with sodium or
potassium hydroxide.
Mg2+(aq)
+ 2KOH(aq) → Mg(OH)2(s) + 2K+ (3.1)
Calcium, strontium and barium oxides react
exothermically with water to form the corresponding hydroxides:
CaO(s) + H2O(l)
→ Ca(OH)2(s)
SrO(s) + H2O(l)
→ Sr(OH)2(s)
BaO(s) + H2O(l)
→ Ba(OH)2(s) (3.2)
Magnesium and calcium hydroxides are sparingly soluble in water
and the resulting aqueous solutions are mildly alkaline. In general, group 2
hydroxides, except Be(OH)2, react as bases, and their water
solubility and thermal stability increase within the group (Mg→Ba).
Earth alkaline halides (MCl2) are
normally found in their hydrated form. Anhydrous beryllium halides are covalent, whereas Mg(II), Ca(II), Sr(II), Ba(II)
halides are ionic. As a result of the ionic bond, the later halides have
typically high melting points and they are sparingly soluble in water.
Additionally, MgCl2, MgBr2, MgI2 are
hygroscopic. Anhydrous calcium chloride also has a strong affinity for water
and is typically used as a drying agent.
Hygroscopic compounds are substances that absorb water from the surrounding air but do not become a liquid.
Related Topics
