Potassium and its clinical application
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Alkali Metals
Potassium has atomic number 19 and the chemical symbol K, which is derived from its Latin name ‘kalium’.
Potassium
and its clinical application
Potassium has atomic number 19 and the chemical symbol K, which
is derived from its Latin name ‘kalium’. Potassium was first isolated from
potash, which is potassium carbonate (K2CO3). Potassium
occurs in nature only in the form of its ion (K+) either dissolved
in the ocean or coordinated in minerals because elemental potassium reacts
violently with water. Potassium ions are essential for the human body and are
also present in plants. The major use of K+ can be found in
fertilisers, which contains a variety of potassium salts such as potassium
chloride (KCl), potassium sulfate (K2SO4) and potassium
nitrate (KNO3). KCl is also found in table salt, whereas potassium
bromate (KBrO3) is an oxidising agent and is used as flour improver.
Potassium bisulfite (KHSO3) can be used as a food preservative in
wine and beer.
1. Biological importance of potassium ions in the human body – action potential
The so-called action potential occurs in a variety of excitable
cells such as neurons, muscle cells and endocrine cells. It is a short-lasting
change of the membrane potential and plays a vital role in the cell-to-cell
communi-cation. In animal cells, there are two types of action potential. One
type is produced by the opening of calcium ion (Ca2+) channels and
this is longer lasting than the second type, namely the Na+-based
action potential.
The Na+/K+-based action potential is
short-lived (only 1 ms) and therefore mostly found in the brain and nerve
cells. Potassium ions are crucial for the functioning of neurons, by
influencing the osmotic balance between the cells and the interstitial fluid.
The concentration of K+ within and outside the cells is regulated by
the so-called Na+/K+-ATPase pump. Under the use of ATP,
three Na+ ions are pumped outside the cell and two K+
ions are actively transported into the cell. As a result, an electrochemical
gradient over the cell membrane is created; the so-called resting potential is
established.
In the case of any cell-to-cell communication, changes of the
membrane reach a specific part of the neu-ron first. As a result, sodium
channels, which are located in the cell membrane, will open. As a result of the
osmotic gradient, which has been established by the Na+/K +-pump,
Na+ ions enter the cytosol and the electrochemical gradient becomes
less negative. Once a certain level, called the threshold, is reached, more sodium channels are opened, and more Na+
ions flow inside the cell – this creates the so-called action poten-tial. The
electrochemical gradient over the cell membrane is thus reversed. Once this
happens, the sodium ion channels close and the potassium channels open. The
concentration of K+ in the cytosol is higher than in the
extracellular fluid, and therefore potassium ions leave the cell. This allows
the cell to shift back to its resting membrane potential. As the membrane
potential approaches the resting potential, all voltage-gated K+
channels open. In actual fact, the membrane repolarises beyond the resting
potential; this is known as hyperpolarisation.
The last step is now to reintroduce the initial balance of sodium and potassium
ions. This means that the Na+/K+-pump
transports Na+ actively out of the cytosol and K+ into
it. As a result, the initial steady state is reinstated (Figure 2.18).
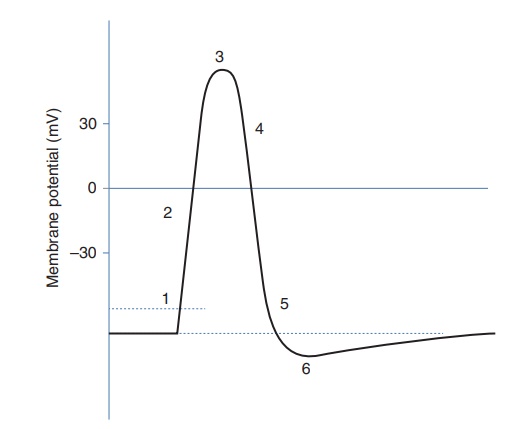
Figure 2.18 Action potential. (1) Threshold of excitation. Na+ channels open and allow Na+ to enter the cell. (2) K+ channels open and K+ leaves the cell. (3) No more Na+ enters cell. (4) K+ continues to leave the cell. This causes the membrane to return to resting potential. (5) K+ channels close and Na+ channels resent. (6) Hyperpolarisation
2. Excursus: the Nernst equation
As previously outlined, the electric potential
across a cell membrane is created by the difference in ion concen-tration
inside and the outside the cell. The Nernst
equation is an important equation that allows the calculation of the
electric potential for an individual ion.
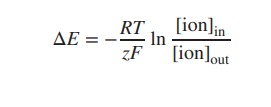
Looking at the Nernst equation, R is the gas constant, T
is the temperature in kelvin, F is
the Faraday constant and z is the net
charge of the ion. [ion]in and [ion]out are the
concentration of the particular ion inside and outside the cell. The equation
can also be expressed as log10. Furthermore, at room temperature (25
∘C),
the term −RT/F can be seen as a constant, and the Nernst equation can be
simplified:
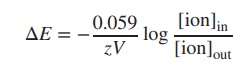
The Nernst equation can also be used to
calculate the overall potential in a redox equation by using the ion
concentrations of both half-equations. This also allows the calculation of the
reduction potential E of two different
ions of varying concentration. The Nernst equation can be expanded to the
following equation, where [Red] and
[Ox] represent the concentrations of the reductant and oxidant (Ox + e−
→ Red):
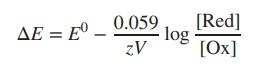
In order to calculate the reduction potential ΔE of two
different ions of varying concentration, the Nernst equation can be
expanded to the following equation:
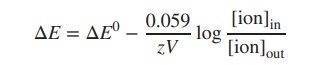
It is also possible to calculate the overall
electric potential across the cell membrane by taking several different ions
into account. The so-called Goldman equation, which will not be further
illustrated in this book, can be used to calculate this value.
3. Potassium salts and their clinical application: hypokalaemia
In the human body, 95% of the K+ can be found inside
the cells, with the remaining 5% mainly circulating in the blood plasma . This
balance is carefully maintained by the Na+/K+ pump, and
imbalances, such as seen in hypo or hyperkalaemia, can have serious
consequences.
Hypokalaemia is a potentially serious condition where the
patient has low levels of K+ in his/her blood plasma. Symptoms can
include weakness of the muscles or ECG (electrocardiogram) abnormalities.
Mostly, hypokalaemia can be a result of reduced K+ intake caused by
GI disturbance, such as diarrhoea and vomit-ing, or increased excretion of K+
caused by diuresis. Hypokalaemia is often found in patients treated with
diuretics such as loop diuretics and thiazides. These classes of drugs increase
the secretion of Na+ in the nephrons in order to increase water
excretion. Unfortunately, they also increase the excretion of K+ and
lead to hypokalaemia. In contrast, potassium-sparing diuretics actively
preserve potassium ions, and patients treated with loop diuretics or thiazides
often receive also potassium-sparing diuretics [3a].
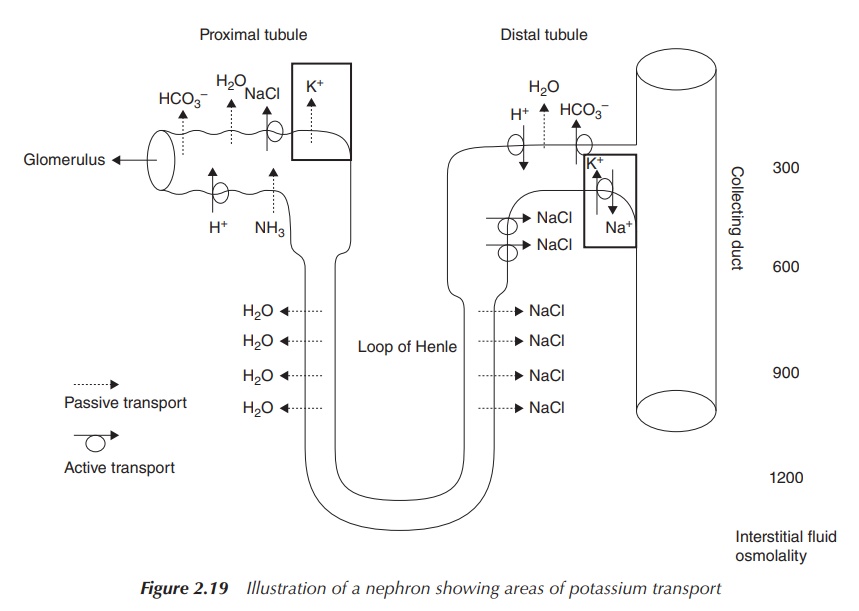
Potassium ions are excreted via the kidneys. Within the kidneys,
∼150–180
l of plasma is filtered every day through the glomerulus, which is part of the
nephron, in order to produce urine. As previously described, the filtration
process is followed by a series of processes along the nephron, where a variety
of ions are secreted and re-absorbed in order to regulate plasma imbalances and
manage the urine volume (Section 2.3.1). K+ is passively secreted at
the proximal tubule and also moves into the interstitial fluid via a
counter-flow process to Na+ mainly at the distal tubule (Figure
2.19).
Oral supplementation in form of potassium salts is especially necessary in patients who take anti-arrhythmic drugs, suffer from renal artery stenosis and/or severe heart failure or show severe K+ losses due to chronic diarrhoea or abusive use of laxatives.
Regulation of the plasma
K+ level may also be required in the care of elderly patients when
the K+ intake is reduced as a result of changing dietary habits, but
special attention has to be given to patients with renal insufficiency because
K+ excretion might be reduced. Potassium salts are preferably given
as liquid preparations, and KCl is the preferred salt used. Other
potassium-based salts can be used if the patient is at risk of developing
hyperchloraemia – increased chloride plasma levels. Typically potassium salts
are dissolved in water, but the salty and bitter taste makes them difficult to
formulate. Oral bicarbonate solutions such as potassium bicarbonate are
typically given orally for chronic acidosis states – low pH of the blood
plasma. This can be again due to impaired kidney function. The use of potassium
bicarbonate for the treatment of acidosis has to be carefully evaluated, as
even small changes of the potassium plasma levels can have severe consequences
(Figure 2.20) [3a].
Potassium citrate is used in the United Kingdom as an
over-the-counter drug for the relief from discomfort experienced in mild
urinary-tract infections by increasing the urinary pH. It should be not given
to men if they experience pain in the kidney area (risk of kidney stones) or if
blood or pus is present in the urine. Also, patients with raised blood pressure
or diabetes should avoid taking potassium citrate without consultation with
their general practitioner (GP). Caution is generally advised to patients with
renal impairment, cardiac problems and the elderly [3a].
4. Adverse effects and toxicity: hyperkalaemia
The therapeutic window for K+ in the blood plasma is
very small (3.5–5.0 mmol), and especially hyper-kalaemia, an increased level of
K+ in the plasma, can lead to severe health problems . Potassium
salts can cause nausea and vomiting and in extreme cases can lead to small
bowel ulcerations. Acute severe hyper-kalaemia is defined when the plasma
potassium concentration exceeds 6.5 mmol/l or if ECG changes are seen. This can
lead to cardiac arrest, which needs immediate treatment. Treatment options
include the use of calcium gluconate intravenous injections, which minimises
the effects of hyperkalaemia on the heart. The intravenous injection of soluble
insulin promotes the shift of potassium ions into the cells. Diuretics can also
be used to increase the secretion of K+ in the kidneys, and dialysis
can be a good option if urgent treat-ment is required. Ion-exchange resins,
such as polystyrene sulfonate resins, may be used in mild to moderate
hyperkalaemia to remove excess potassium if there are no ECG changes present.
As previously mentioned, especially in patients suffering from kidney diseases
or end-stage renal failure, the potassium levels have to be monitored very
carefully and corrected if necessary. Potassium excretion is likely to be
disturbed, and a build-up of potassium in the blood plasma may trigger a
cardiac arrest [3a].
Potassium salts are also available in the form
of tablets or capsules for oral application especially as nonpre-scription
medicine. Usually, their formulation is designed to allow the potassium ions to
be slowly secreted, because very high concentrations of K+ are known
to be toxic to tissue cells and can cause injury to the gastric mucosa.
Therefore, nonprescription potassium supplement pills are usually restricted to
<100 mg
of K+ .
Related Topics
