Alkali metal ions
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Alkali Metals
This group of elements belongs to the so-called s-block metals as they only have one electron in their outer shell, which is of s type.
Alkali
metal ions
This group of elements belongs to the
so-called s-block metals as they only have one electron in their outer shell,
which is of s type. The chemistry of the metals is characterised by the loss of
this s electron to form a monocationic ion M+, which results from
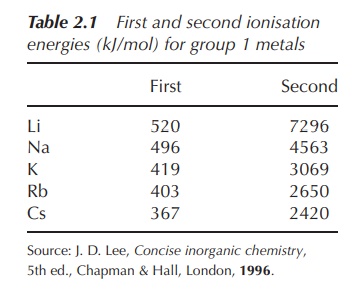
the relatively low ionisation energy of this electron (Table 2.1).
The
term ionisation energy (IE) is
defined as the energy that is required to remove the outer electron of an atom
or molecule. The tendency to lose the outer electron is directly correlated to
the ionisation energy – the lower the ionisation energy, the easier the removal
of the electron.
Within
the group of alkali metals, the ionisation energy for the removal of the outer
electron decreases as a result of the increasing distance of this electron from
the nucleus.
The loss of the outer s electron within the
group of alkali metals results in the formation of the M+ ion as
mentioned. Consequently, most of the compounds of group 1 elements tend to be
ionic in nature and form salts. In all pharmaceutical applications, only the
salts of alkali metals are used, as most of the pure metals react violently
with water.
1.
Extraction of alkali metals: an introduction to redox chemistry
The main sources for Na and K are rock salts. Both elements do
not naturally occur in their elemental state. In contrast, Li, Rb and Cs have
small natural abundances, but also occur as rock salts. As previously
mentioned, only artificial isotopes of Fr are known.
Sodium is manufactured by the so-called Downs’
process, which is the electrolysis of molten NaCl and represents the major
production process for sodium metal and is also a minor source of industrial
chlorine. The process is based on a redox reaction where the reduction of
liquid Na+ to liquid Na takes place at the cathode and the oxidation
of liquid chloride (Cl−) to chlorine (Cl2) gas at the
anode:

Reduction at the cathode ∶ Na+(l)+
e− → Na(l)
Oxidation at the anode ∶ 2Cl−(l)
→ Cl2(g) + 2e−
----------------------------
Overall redox reaction ∶ 2Na+(l)
+ 2Cl−(l)→ 2Na(l) + Cl2(g)
Molten NaCl is used as the electrolyte medium within the electrolytic Downs’ process and CaCl2 is added in order to decrease the operating temperature. The melting point of NaCl is 800 ∘C, whereas the addition of CaCl2 lowers it to around 600 ∘C. The design of the electrolysis cell is crucial in order to prevent oxidation and hydrolysis of freshly produced sodium and recombination to NaCl. Chlorine (Cl2) is produced at the positive graphite anode and captured in form of gas, whereas Na+ is reduced to liquid sodium at the negative iron cathode and collected in its liquid form.
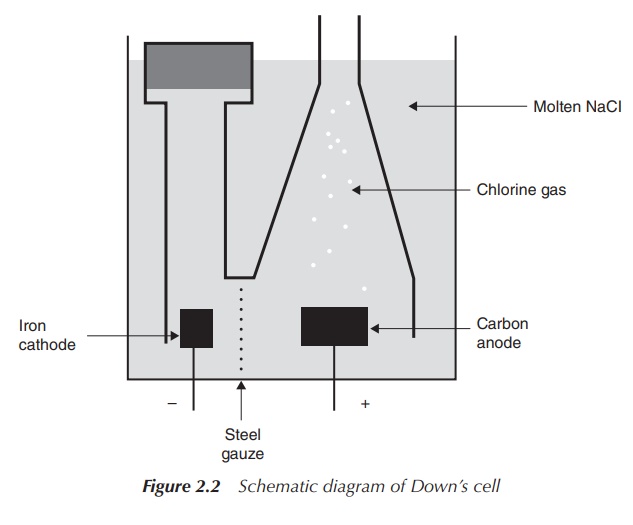
The anode is defined as the electrode at which the oxidation
takes place, which, means in this example, that the anode is positively
charged. Conversely, the cathode is defined as the place where the reduction
takes place and therefore it is negatively charged in electrolysis processes.
Note that the same definition for electrodes applies in a galvanic cell (e.g.
in batteries), but as a result of the electron flow the anode has a negative
charge whereas the cathode is positively charged (Figure 2.2).
Lithium can be isolated in a similar
electrolytic process using lithium chloride (LiCl). Spodumene (LiAlSi2O6)
is the main source for LiCl, which is reacted to LiOH by heating with CaO and
subsequent conversion to LiCl prior to the electrolysis. Potassium can also be
obtained via electrolysis of KCl, but there are more efficient methods, for
example, the reaction to a Na-K alloy which can be subsequently distilled.
2.
Excursus: reduction–oxidation reactions
The term redox (short for reduction–oxidation)
reaction describes all chemical reactions in which atoms change their oxidation
number. Redox reactions have many applications, which can range from industrial
processes (see Downs’ process) to biological systems, for example, oxidation of
glucose in the human body.
A redox reaction contains the two
‘half-reactions’, namely reduction and oxidation, which are always one set;
this means that there is never a reduction reaction without an oxidation.
Redox reactions are a family of reactions that consist of
oxidation and reduction reactions. These are always a matched set, that is, there is no oxidation reaction
without a reduction reaction. They are called half-reactions, as two
half-reactions are needed to form a redox reaction. Oxidation describes the
loss of electrons (e−), whereas the term reduction is used for the gain of electrons
(e−).
•
Reduction:
Describes the gain of electrons or
the decrease in the oxidation state
of an atom or molecule.
•
Oxidation:
Describes the loss of electrons or
the increase in the oxidation state
of an atom or molecule.
Reduction ∶ Fe → Fe2+ + 2e−
Oxidation ∶ Cu2+ → Cu + 2e−
Overall redox reaction ∶ Fe +
Cu2+ → Fe2+ + Cu (2.1)

Equation 2.1 gives an example for a simple redox reaction.
The terms oxidant
and reductant are important terms used
when discussing redox equations. An oxidant is defined as the atom or molecule
that oxidises another compound and in turn is reduced itself. Reductants are
molecules or atoms that reduce other compounds and as a result are oxidised
themselves. Within a redox equation, there is always the oxidant–reductant pair
present.
Reduction ∶ Oxidant
+ e− → Product
Decrease in oxidation state (gain of
electrons)
Oxidation ∶ Reductant
→ Product
+ e−
Increase in oxidation state (loss of
electrons)
Equation 2.2 shows the involvement of an
oxidant and a reductant in redox reactions.
2.1 The oxidation state
It is actually not precisely correct to
describe oxidation/reduction reactions as loss/gain of electrons. Both
reactions are better referred to the changes in oxidation state rather than to
the actual transfer of electrons; there are reactions classified as redox
reaction even though no transfer of electrons takes place.
The oxidation state of an atom describes
how many electrons it has lost whilst interacting with another atom compared to
its original state (in its elemental form). The oxidation state is a
hypothetical charge that an atom would have if all bonds within the molecule
are seen as 100% ionic. When a bond is formed, it is possible for an atom to
gain or lose electrons depending on its electronegativity. If an atom is
elec-tropositive, it is more likely to take electrons away from another atom,
and vice versa.
The
oxidation number relates to the number of electrons that has been allocated to
each atom; that is, if an atom has gained an electron, its oxidation state is
reduced by 1 and therefore this atom has the oxidation state of –I.
IUPAC has defined the oxidation state as
follows: A measure of the degree of oxidation of an atom in a substance. It is
defined as the charge an atom might be imagined to have when electrons are
counted according to an agreed-upon set of rules:
1.
The oxidation state of a free element
(uncombined element) is zero.
2.
For a simple (monatomic) ion, the oxidation
state is equal to the net charge on the ion.
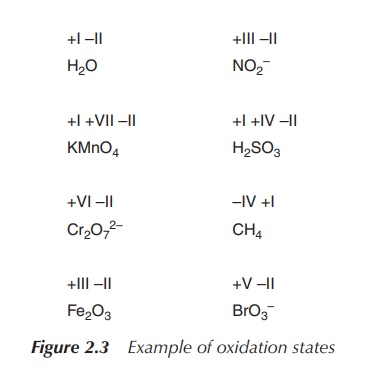
3. Hydrogen has an oxidation state of 1 and oxygen has an oxidation state of −2 when they are present in most compounds (exceptions to this are that hydrogen has an oxidation state of −1 in hydrides of active metals, e.g. LiH, and oxygen has an oxidation state of −1 in peroxides, e.g. H2O2).
4. The algebraic sum of the oxidation states
of all atoms in a neutral molecule must be zero, whilst in ions the algebraic
sum of the oxidation states of the constituent atoms must be equal to the
charge on the ion. For example, the oxidation states of sulfur in H2S,
S8 (elementary sulfur), SO2, SO3 and H2SO4
are, respectively: −2, 0, +4, +6 and +6. ‘The higher the oxidation state of a
given atom, the greater its degree of oxidation; the lower the oxidation state,
the greater its degree of reduction (Figure 2.3)’ .
2.2 How to establish a redox equation?
In order to describe the overall redox
reaction, the establishing and balancing of the half-reaction is essential. In
aqueous reactions, generally electrons, H+, OH− and H2O
can be used to compensate for changes, whereas it has to be kept in mind
whether the reaction takes place under acidic or basic conditions. There are
five steps to follow in order to successfully establish a redox equation:
Example
Determine
the redox equation for the reaction of MnO4− to Mn2+ and Ag to Ag+ under acidic conditions.
1. Determine the oxidation state of each
element involved (oxidation number is stated in Roman numbers):
+VII −II +II
MnO4− → Mn2+
0 +I
Ag → Ag+
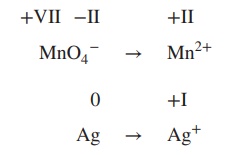
2. Add electrons to the equation; the number
of electrons must reflect the changes in the oxidation state:
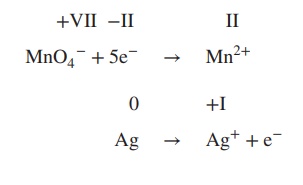
3. Determine which reaction is oxidation or
reduction:
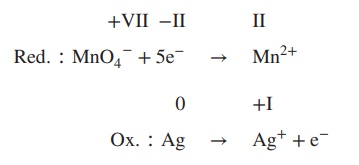
4. Balance reduction and oxidation reaction by
using either H+/H2O (acidic conditions) or OH−/H2O
(basic conditions), depending on the reaction conditions, which can be acidic
or basic (in this example: acidic conditions):
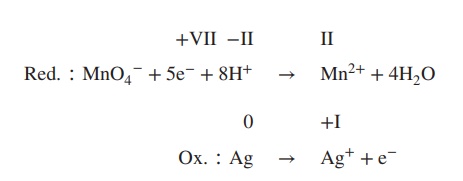
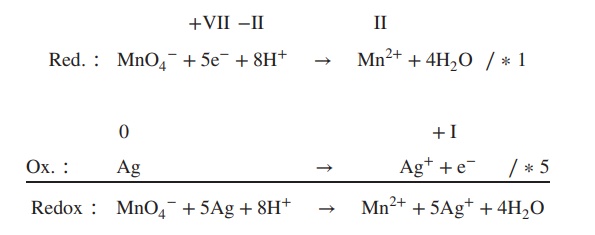
5. Formulate the redox equation keeping in
mind that the number of electrons has to be equal in the reduc-tion and
oxidation reactions. If necessary, the oxidation reaction has to be multiplied
by the number of electrons in the reduction step, or vice versa.
Within the above example, the reaction
conditions are stated as being acidic. A similar example using basic conditions
is stated below and hydroxyl ions and water (OH−/H2O) are
used in order to balance the half-reactions.
Example
Determine
the redox equation for the reaction of MnO4− to MnO2 and Fe2+ to Fe3+ under basic conditions.
1. Determine the oxidation state of each
element involved:
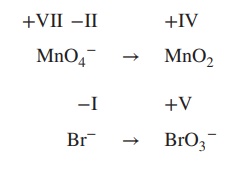
2. Add electrons to the equation; the number
of electrons must reflect the changes in the oxidation state:
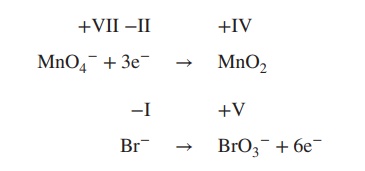
3. Determine the redox and the oxidation
reaction:
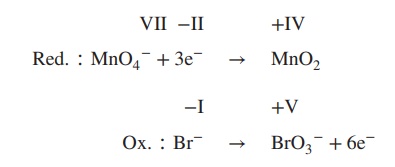
4. Balance reduction and oxidation reaction by
using OH−/H2O:
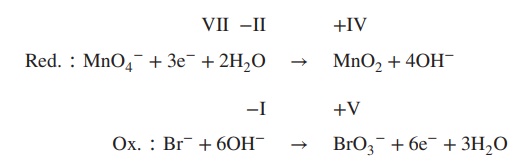
5. Formulate the redox equation:
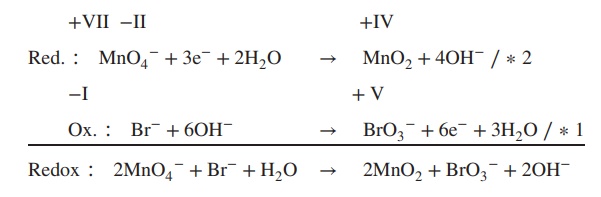
Redox ∶ 2MnO4− + Br− + H2O → 2MnO2 + BrO3− + 2OH−
2.3 How to calculate the redox potential
The spontaneous reaction of Zn(s) metal with Cu2+(aq)
from a Cu(II) solution is the prime example for a redox reaction.
Zn(s) + Cu2+(aq) → Zn2+(aq)
+ Cu(s)
In order to calculate the overall redox
potential, it is important to formulate the individual half-equations as
reductions first. The standard reduction potential Ered0 for each reaction can be taken from
standard tables:
Cu2+(aq) + 2e−
→ Cu(s)
E0red= +0.340V
Zn2+(aq) + 2e−
→ Zn(s)
E0red=
−0.763V
The half-equation with the more positive value
is the reduction, whilst the other reaction is the oxidation. In this example,
Cu2+ will be reduced to Cu whilst Zn(s) metal will be
oxidised to Zn2+. The standard potential E0 of an
oxidation half-equation is the negative value of Ered0.
Reduction ∶ Cu2+ (aq) + 2e− → Cu(s)
Ered0 = +0.340V
Oxidation ∶ Zn(s) → Zn2+(aq)
+ 2e− E0ox =
−(−0.763V)
In order to calculate the voltage produced by
an electrochemical cell (Ecell0),
the potentials of all half-equations are added up.
Ecell0 = Ered0 + Eox0
For the example above ∶ Ecell0
= 0.340 V
+ [−(−0.763
V)] = 1.103 V
If you deal with more complicated redox reactions and half-equations have to be multiplied because of the different numbers of electrons in each half-equation, the standard potential E0 will not be affected by these coefficients. Furthermore, it is important to note that the above calculations can be used only if both reaction partners are present in the same concentration. Concentration can have an effect, and the overall potential is then calculated by using the so-called Nernst equation.
Table 2.2 Table of
standard reduction potential under acidic conditions at25 ∘C
E0
(V)
Li+
+ e- → Li −3.05
K+
+ e- → K −2.93
Ba2+
+ 2e- → Ba −2.90
Na+
+ e- → Na −2.71
Mg2+
+ 2e- → Mg −2.37
Al3+
+ 3e- → Al −1.66
Mn2+
+ 2e- → Mn −1.18
Ga3+
+ 3e- → Ga −0.53
Tl+
+ e- → Tl −0.34
Fe2+
+ 2e- → Fe −0.44
Cr3+
+ 3e- → Cr −0.74
Cr3+
+ e- → Cr2+ −0.41
Ni2+
+ 2e- → Ni −0.25
Cu2+
+ e- → Cu+ +0.15
Cu2+
+ 2e- → Cu +0.35
Cu+
+ e- → Cu +0.50
I2
+ 2e- → 2I - +0.54
Fe3+
+ e- → Fe2+ +0.77
Br2
+ 2e- → 2Br- +1.07
MnO2
+ 2e- → Mn2+ +1.23
Cl2
+ 2e- → 2Cl- +1.36
MnO4-+
5e- → Mn2+ +1.54
F2
+ 2e- → 2F- +2.65
3. Chemical
behaviour of alkali metals
Most alkali metals have a silvery white appearance with the
exception of caesium which is golden yellow. They are all soft metals and
typically can be cut with a knife. The softness of the metal increases within
the group; caesium is the softest of the alkali metals.
Alkali metals are generally very reactive and oxidise in the
air. The reactivity increases within the group, with lithium having the lowest
reactivity and caesium the highest. Therefore, all alkali metals except lithium
have to be stored in mineral oil. Lithium as an exception is normally stored
under inert gas such as argon. Nevertheless, lithium, sodium and potassium can
be handled in air for a short time, whereas rubidium and caesium have to be
handled in an inert gas atmosphere.
All alkali metals react violently with water
with the formation of the metal hydroxide and hydrogen. Again, lithium is the
least reactive alkali metal and reacts ‘only’ quickly with water, whereas
potassium, rubidium and caesium are more reactive and react violently with
water.
2Li(s) + 2H2O → 2LiOH
+ H2(g) ↑
(2.3)
In terms of their pharmaceutical applications, alkali metals are
not directly useable mainly because of their reaction behaviour in aqueous
media. Nevertheless, alkali metal halides, some oxides, carbonates, citrates
and other salts are of medicinal interest. NaCl and KCl solutions are important
as oral rehydration salts, and KCl can also be used to treat potassium
depletion.
In general, alkali metal halides can be
prepared by the direct combination of the elements, that is, the reaction of an
alkali metal with halogens. Alkali metal halides are very soluble in water,
which is important for a potential pharmaceutical application, and partly
soluble in organic solvents.
2M+X2 →2MX (2.4)
Alkali metal oxides can be synthesised by
heating alkali metals in an excess of air. Thereby, the oxide, peroxide or
superoxide formation can be observed depending on the metal.
4Li + O2 → 2Li2O
oxide formation
2Na + O2 → Na2O2
peroxide formation
K+O2 → KO2 superoxide formation (2.5)
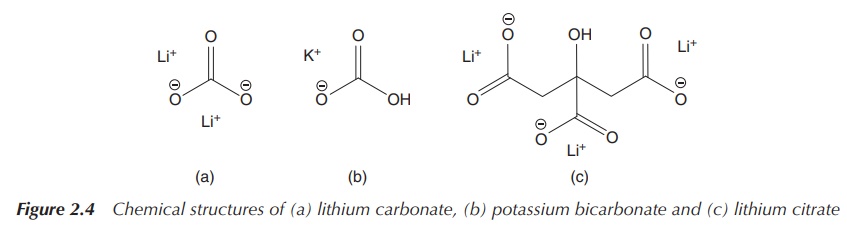
Alkali metal carbonates and bicarbonates have wide-ranging
pharmaceutical applications. Lithium bicarbonate or citrate is used in the treatment
of BD, whereas potassium bicarbonate or citrate is used in over-the-counter
drugs as active pharmaceutical ingredients (APIs) against urinary-tract
infections (increasing the pH of the urine) in the United Kingdom. Their
solubility is highly dependent on the metal and varies from sparingly soluble
(e.g. Li2CO3) whereas others are very soluble (Figure
2.4).
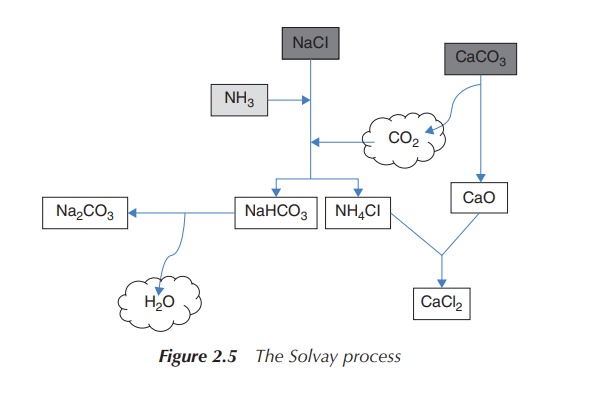
Sodium carbonate is produced by the so-called
Solvay process – one of the most important industrial pro-cesses, which was
developed in the 1860s by Ernest Solvay. Na2CO3 has
extensive uses ranging from glass production to its application as a water
softener. The starting materials, which are NaCl and CaCO3 (lime
stone), are inexpensive and easily available.
2NaCl + CaCO3 → Na2CO3
+ CaCl2 (2.6)
CaCO3 is heated to around 1000 ∘C, when it is
converted to CO2 and CaO (quicklime).
CaCO3 → CO2
+ CaO
CO2 is then passed through an aqueous solution of NaCl and NH3 (ammonia). NH3 buffers the solution at a basic pH and NaHCO3 (sodium bicarbonate) precipitates out of this solution. NaHCO3 is less water soluble than NaCl at a basic pH. Without the addition of NH3, the solution would be acidic, as HCl is produced as a by-product.
NaCl + CO2 + NH3 + H2O
→ NaHCO3
+ NH4Cl (2.8)
NaHCO3 is filtered, and the
remaining solution of NH4Cl reacts with the CaO from step 1. The
produced CaCl2 is usually used as road salts, and NH3 is
recycled back to the initial reaction of NaCl (reaction step 2).
2NH4Cl + CaO → 2NH3
+ CaCl2 + H2O (2.9)
In a final step, NaHCO3 is
converted to Na2CO3 (sodium carbonate) by calcination
(heating to 160–230 ∘C)
with the loss of water.
2NaHCO3 → Na2CO3
+ H2O + CO2 (2.10)
Again, the CO2 can be recycled and reused within the
process. This means that the Solvay process consumes only a very small amount
of NH3 and the only ingredients are NaCl and CaCO3
(Figure 2.5).
In terms of pharmaceutical applications, the
various salts of sodium, lithium and potassium have the widest use and are
discussed in detail in the following sections. These applications range from
the use of sodium and potassium salts in dehydration solutions in order to
restore replenished mineral balances to the treatment of BD with simple lithium
salts.
Related Topics
